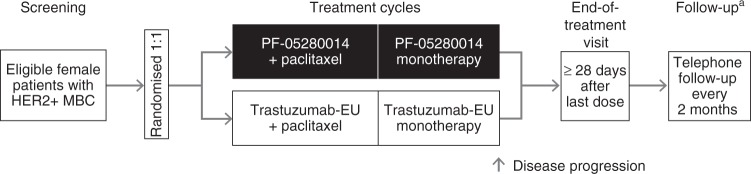
Fig. 1.
Study design. PF-05280014 or trastuzumab-EU: administered weekly (4 mg/kg loading dose on Cycle 1 Day 1; subsequent doses 2 mg/kg) on Days 1, 8, 15 and 22 of each 28-day cycle during the paclitaxel administration period and until at least Week 33. Following completion of the paclitaxel administration period and beginning no earlier than Week 33, the PF-05280014 or trastuzumab-EU regimen could be changed to 6 mg/kg every 3 weeks. Treatment with PF-05280014 or trastuzumab-EU could continue until disease progression. Paclitaxel: administered on Days 1, 8 and 15 of each 28-day cycle (starting dose 80 mg/m2, with provision for dose reduction). In the absence of disease progression or unacceptable toxicity in the judgement of the investigator, paclitaxel treatment was continued for ≥ 6 cycles or until maximal benefit of response was obtained. On days when both treatments were administered, the order of administration was PF-05280014 or trastuzumab-EU infusion followed by paclitaxel infusion. aFollow-up to assess survival status continued until death or until 1 year from randomisation and ≥ 6 months following last dose of study treatment. HER2+ human epidermal growth factor receptor 2-positive, MBC metastatic breast cancer, trastuzumab-EU reference trastuzumab sourced from the European Union