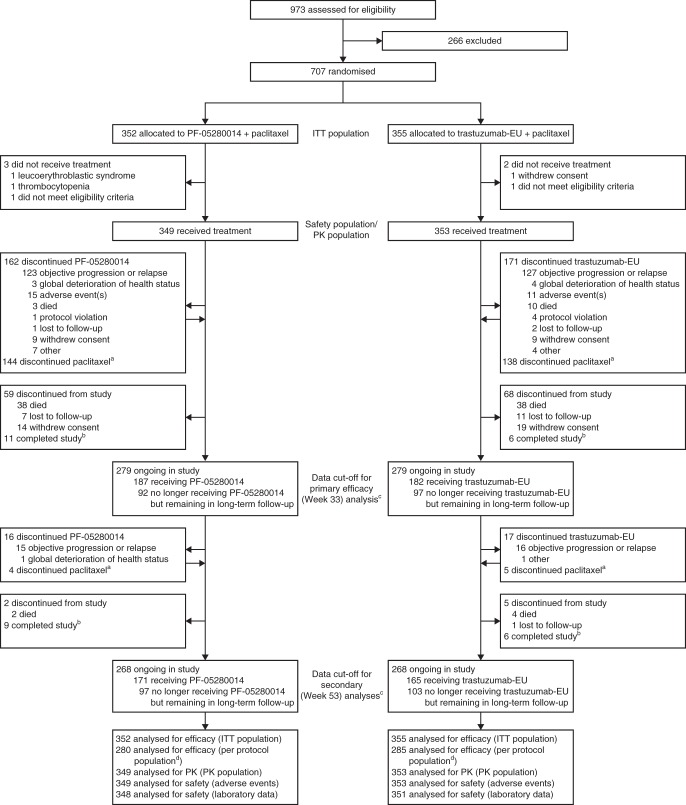
Fig. 2.
Study profile. Primary efficacy analysis based on a data cut-off date (24 August 2016) when all patients had either completed Week 33 tumour assessments or discontinued study treatment earlier. Secondary analyses based on a data cut-off date (11 January 2017) when all patients had either completed Week 53 tumour assessments or discontinued study treatment earlier. aNumber does not include patients who completed treatment with paclitaxel per protocol. bPatients who completed all follow-up as required by the protocol. cStatus at time of data cut-off, using data up to 378 days post-randomisation. dReasons for exclusion from the per protocol population were comparable between the groups. The most frequent reason for exclusion was “no measurable disease at baseline per central reviewer” (31 [8.8%] patients in the PF-05280014 group vs. 25 [7.0%] patients in the trastuzumab-EU group), followed by “missing, not evaluable or equivocal HER2 status by central laboratory” (27 [7.7%] vs. 25 [7.0%] patients). HER2 human epidermal growth factor receptor 2, ITT intent-to-treat, PK pharmacokinetics, trastuzumab-EU reference trastuzumab sourced from the European Union