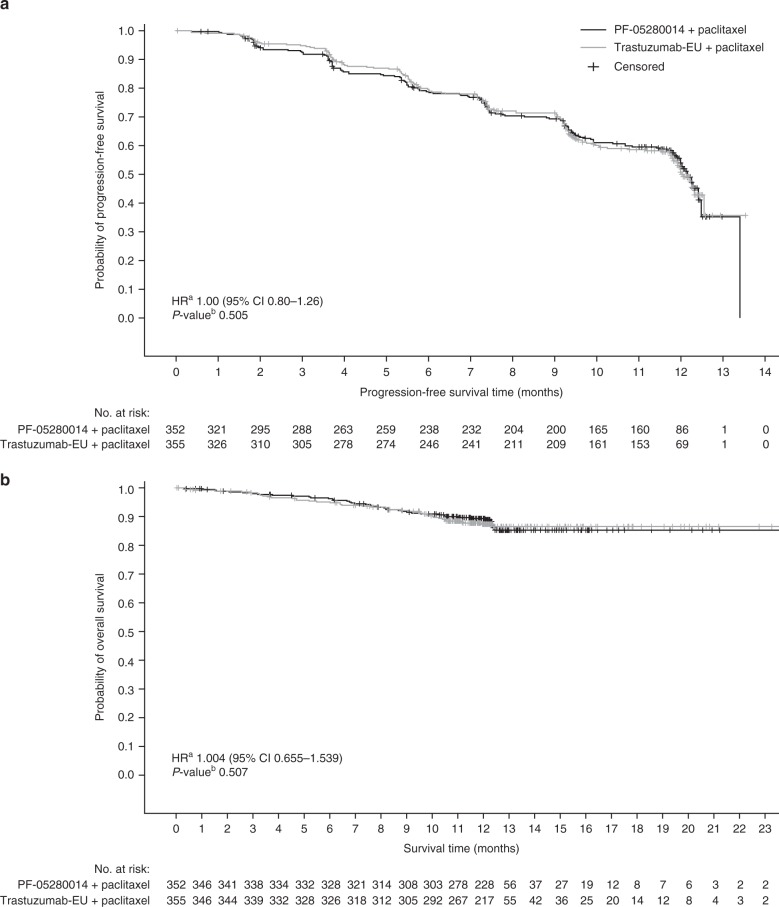
Fig. 3.
Kaplan–Meier plots of (a) progression-free survival (based on central radiology assessments) and (b) overall survival (ITT population) – Week 53 analysis. aHRs from a Cox proportional hazards model with prior trastuzumab exposure and oestrogen receptor status as strata. Assuming proportional hazards, an HR < 1 indicates an increased hazard rate for trastuzumab-EU plus paclitaxel; an HR > 1 indicates an increased hazard rate for PF-05280014 plus paclitaxel. bOne-sided P value from stratified log-rank test. CI confidence interval, HR hazard ratio, ITT intent-to-treat, trastuzumab-EU reference trastuzumab sourced from the European Union