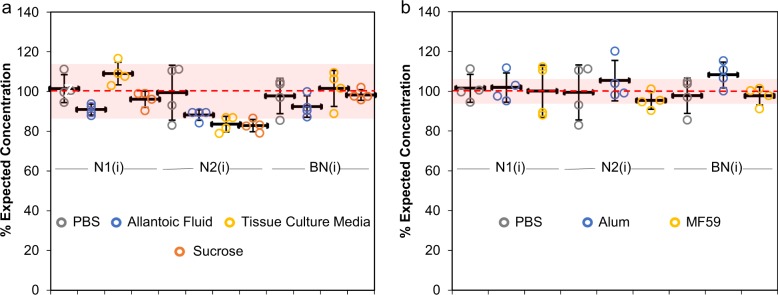
Fig. 5.
Quantification of neuraminidase (NA) by VaxArray Influenza Seasonal NA Potency Assay (VXI-sNA) in the presence of common interfering agents. The following antigens were spiked into phosphate-buffered saline (PBS), allantoic fluid, 40% sucrose, and exhausted Dulbecco’s modified Eagle’s medium (DMEM) + 10% fetal bovine serum (FBS) medium from uninfected Madin–Darby Canine Kidney (MDCK) cells (tissue culture media) to a final concentration of 2 µg/mL and then analyzed by VXI-sNA: H1N1 A/California (CBER, Lot # 76), H3N2 A/Hong Kong (CBER, Lot # 84), and B/Phuket (CBER, Lot # 80). The VXI-sNA measurements for each capture antibody were then divided by the expected concentration of 2 µg/mL and multiplied by 100 to generate “% Expected Concentrations” that were then plotted individually for each replicate a. VXI-sNA potency determination for influenza antigens spiked into common adjuvants such as aluminum hydroxide (alum) and MF59 is shown in b. For each sample, a PBS-negative control was included. For both a and b each data point represents a single replicate. The thick black bar represents the average across the four replicates. Error bars represent the standard deviation across the four replicates for each sample. The red-dotted line represents the 100% expected concentration for each sample. The shaded red region represents 100% expected concentration plus and minus 10% for a and 5% for b