Figure 1.
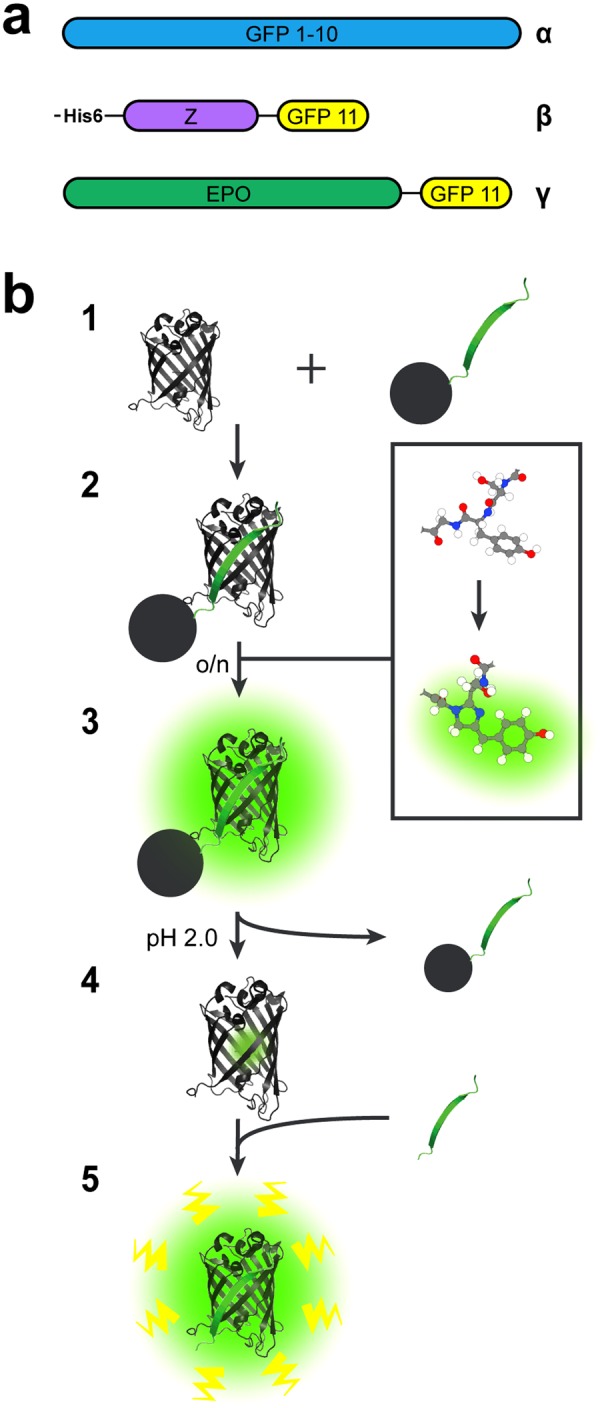
Recombinant proteins used in this study and GFP 1–10 pre-maturation scheme. (a) Schematic representation of the domains present in the proteins used in this study: (α) GFP 1–10 (β) His6-Z_GFP 11, and (γ) EPO_GFP 11. Both His6-Z_GFP 11 and EPO_GFP 11 have a short GS-linkers before GFP 11. (b) Illustration of the GFP 1–10 pre-maturation process on a solid support containing His6-Z_GFP 11. (1) The 11th sheet of GFP fused to the Z domain derived from protein A (His6-Z_GFP 11) is covalently coupled to Sepharose beads. The remaining part of GFP (GFP 1–10) is supplemented to the beads, (2) whereupon the two subunits self-associate and thereby complete the GFP structure. This initiates the relatively slow rearrangement of covalent bonds, which is necessary for the chromophore present in GFP 1–10 to mature. (3) Eventually, GFP 1–10 bound to the beads via GFP 11 will start to fluoresce as their chromophores have been maturated. Unbound GFP 1–10 and other impurities are washed away (4) before the bound GFP 1–10mat is released from GFP 11 by low pH and the eluate is neutralized. (5) The released GFP 1–10mat now comprises a maturated chromophore and is therefore prone to start fluorescing more rapidly when re-encountering GFP 11.
