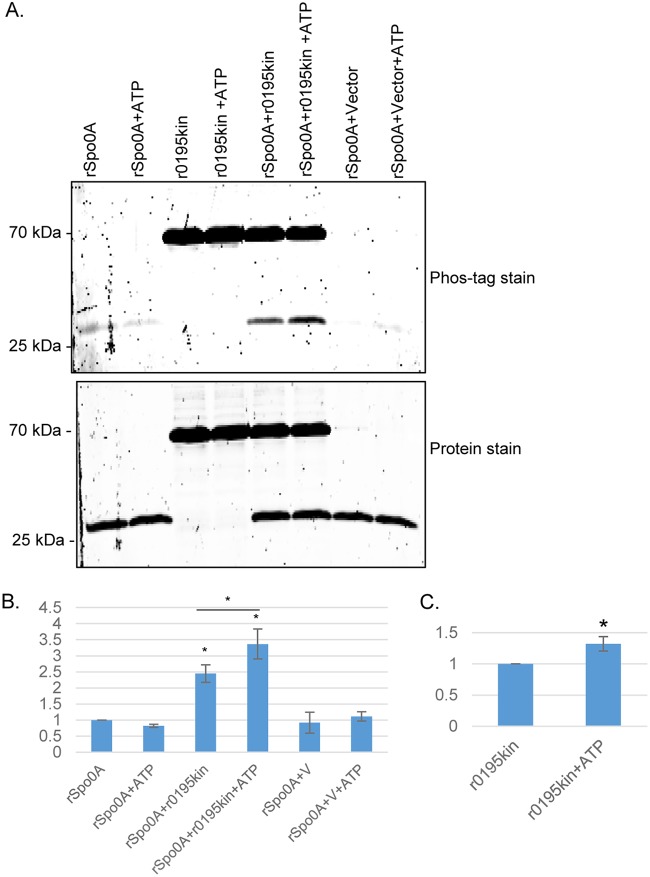
FIG 7.
Phosphotransfer from the recombinant CPR0195 kinase domain rCPR0195kin (r0195kin) to recombinant Spo0A (rSpo0A) in vitro. Purified r0195kin (0.4 µM) was incubated with rSpo0A (4 µM) in phosphotransfer buffer that did or did not contain (as indicated) ATP at room temperature for 1 h. Those samples were then electrophoresed overnight at 4°C on a 15% acrylamide gel containing SDS. (A) The upper photograph shows a gel stained with Phos-Tag phosphoprotein gel stain; the lower photograph shows the same gel stained with eLuminol protein gel stain to show equivalent loading levels of rSpo0A or r0195kin, as appropriate, in different lanes. (B) Quantitative analysis of rSpo0A phosphoprotein levels with or without r0195kin and in the absence or presence of ATP, as indicated. The band intensities of gels were compared by Image J analysis. *, P < 0.05 (in comparison to Spo0A without ATP by ordinary one-way analysis of variance [ANOVA]). #, P < 0.05 (in comparison to r0195kin without ATP; Student's t test). (C) Quantitative analysis of r0195kin phosphoprotein levels in the absence (left) or presence (right) of ATP. The band intensities of gels were compared by ImageJ analysis. *, P < 0.05 (in comparison to r0195kin without ATP; Student's t test). All experiments were repeated three times, and mean values are shown in panels B and C. The error bars indicate standard deviations.