Figure 2.
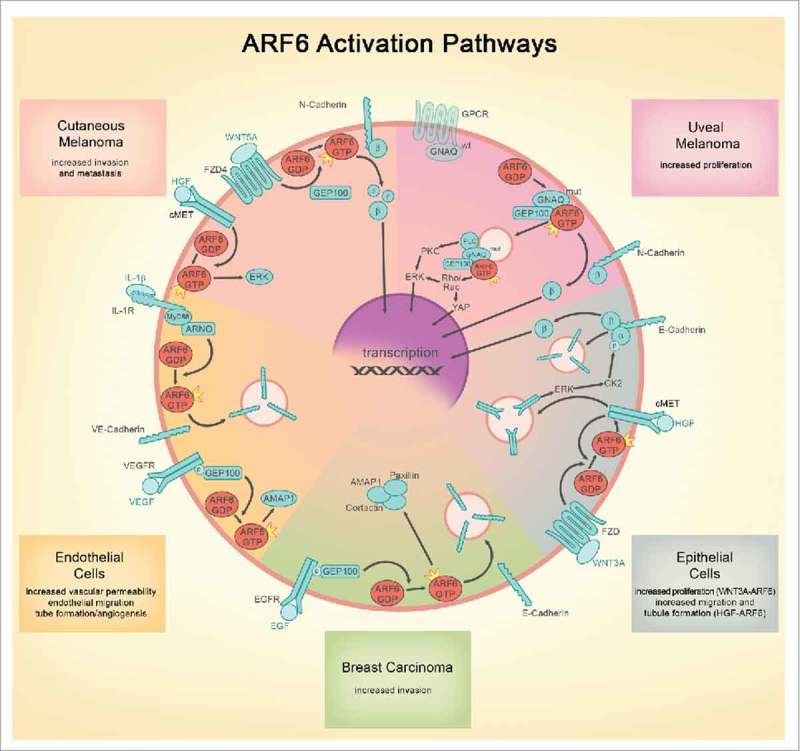
Similarities and differences of ARF6-mediated signaling in various model systems. The following common themes are illustrated: 1) Growth factors, inflammatory cytokines, and WNTs all activate ARF6 to control adherens junction integrity via endocytosis; 2) Both receptor tyrosine kinase and G-protein signaling are coordinated from endosomes by ARF6; and 3) β-catenin junctional and transcriptional function is controlled by WNT-activated ARF6 in non-neoplastic/normal epithelial cells as well as melanoma. ARF6 pathways appear to be divergent in the exact guanine exchange factors engaged to activate ARF6 and in the ultimate cellular phenotype (proliferation vs. barrier function/migration/invasion). Because engagement of ARF6 is a proximal event that is critical for endosomic signaling, ARF6 controls multiple downstream, parallel pathways. For this reason and because ARF6 activation leads to pathologic outcomes in both normal and neoplastic cells, ARF6 is an attractive target for development of therapeutic interventions. FZD = Frizzled, HGF = hepatocyte growth factor, VEGF = vascular endothelial growth factor, EGF = epithelial growth factor, GPCR = G protein coupled receptor, IL-1 = Interleukin 1, PLC = phospholipase C, PKC = protein kinase C, β = β catenin, α = α catenin, wt = wild type, mut = oncogenic mutant.
