Figure 2.
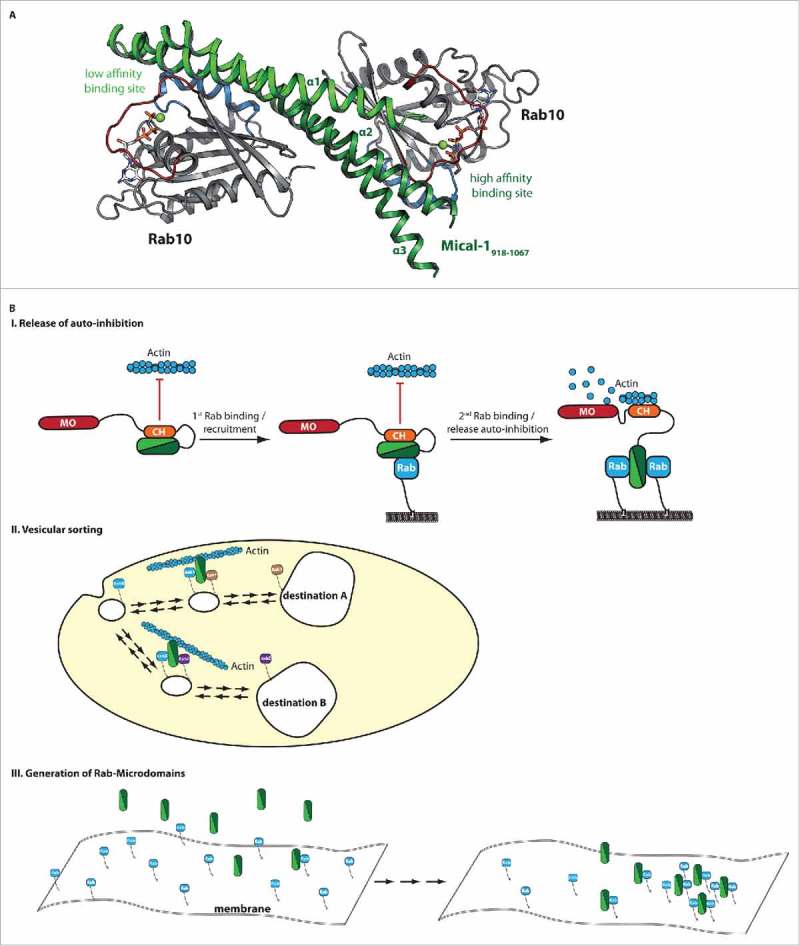
Structure and putative functions of bMERB domains. (A) The X-ray crystallographic structure of Mical-1 in complex with Rab10 revealed two separate Rab binding sites (additional biochemical characterization showed that the binding sites have different affinities to Rab proteins and the corresponding low and high affinity binding sites at the N- and C-terminus, respectively, are indicated).18 (B) Putative functions of bMERB domains are illustrated. These include the release of auto-inhibition by competitive replacement of the auto-inhibitory interaction due to Rab-binding (I.), sorting of vesicular cargo via different specificities of the two separate Rab-binding sites toward certain Rab proteins and establishment of a connection between different Rab compartments (II.) and finally the formation of Rab microdomains on intracellular membranes (III.).
