Figure 4.
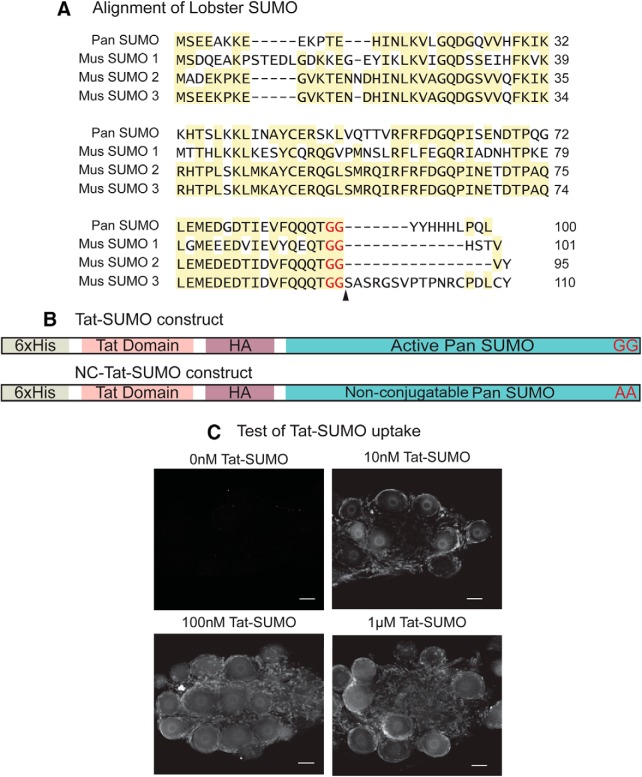
Generating a tool to globally increase SUMOylation in LP. A, Comparison of lobster and mouse SUMOs. SUMO was cloned from spiny lobster, P. interruptus (GenBank accession: MF770707). Aligning the amino acid sequence showed that Panulirus SUMO shares ∼47% identity with mouse SUMO1(GenBank accession: P63166) and ∼67% identity with mouse SUMO2 and 3 (GenBank accession: P61957 and Q9Z172 respectively). Highlighted areas represent regions of conservation. Arrowhead indicates the site where SUMO is cleaved to generate the mature peptide ending in diglycine. B, Tat-HA-His-SUMO construct. Activated Panulirus SUMO (C-terminal diglycine) was subcloned into a Tat-HA-His vector. As a negative control, a plasmid encoding a nonconjugatable Tat-SUMO (NC-Tat-SUMO) ending in a dialanine was also constructed. C, Tat-SUMO is efficiently taken up by pyloric neurons. Recombinant Tat-SUMO peptide was isolated using the plasmid in Figure 4B and standard techniques as detailed in Materials and Methods. Varying concentrations of the peptide (0 nm, 10 nm, 100 nm, and 1 μm) were bath-applied to STG, which contain the pyloric circuit. STGs were fixed and immunostained with anti-Tat. Tat immunoreactivity was not visible in the absence of bath-applied Tat-SUMO (0 nm). When Tat-SUMO was bath-applied, immunoreactivity was clearly visible in the nucleus and cytoplasm of the neurons as well as in the surrounding glial cells. Tat-SUMO uptake appeared to increase in a concentration-dependent manner; 100 nm appeared to be optimal with no detectable improvement at a higher concentration. Scale bar, 50 μm.