Figure 1: Photoredox-catalyzed decarboxylative functionalization as a novel electron transfer mechanism towards site- and chemoselective bioconjugation.
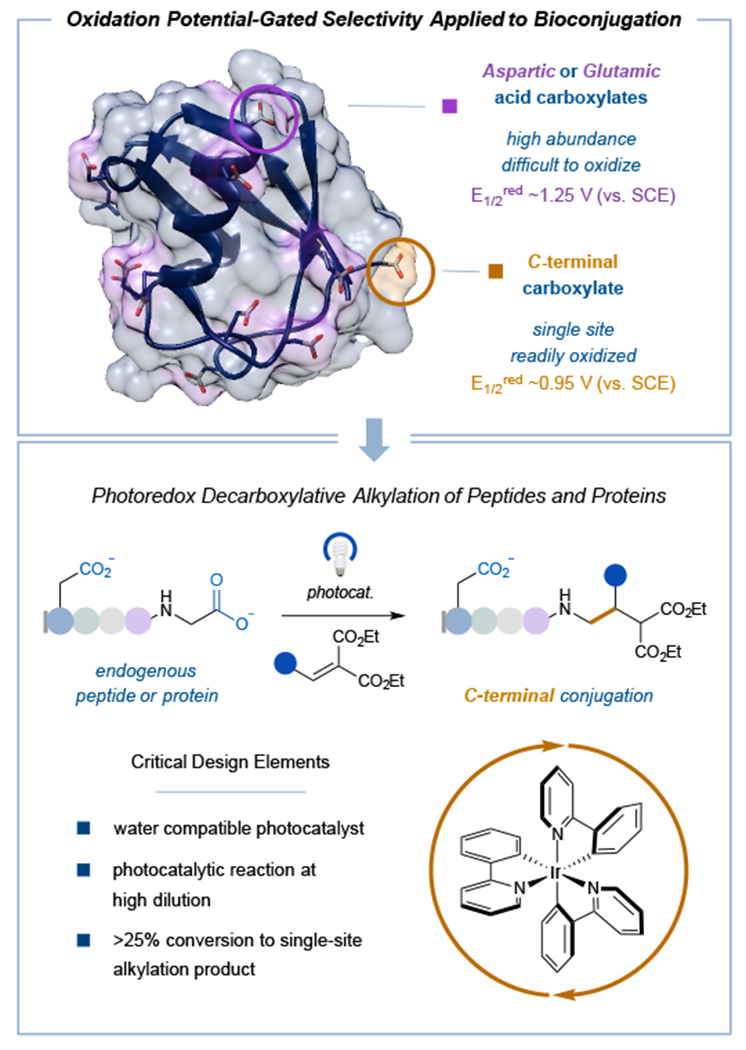
Current methods targeting natural amino acid motifs rely on the intrinsic availability of targeted residues on the protein surface to achieve homogeneously modified products. We postulated that photoredox-catalyzed decarboxylation could capitalize on the inherent differences in oxidation potentials between the more abundant Asp and Glu residues vs. the lone terminal carboxylate to obtain exclusive C-terminal-functionalized products.
