Figure 4. Scope of the photoredox decarboxylative conjugate addition applied to endogenous peptides.
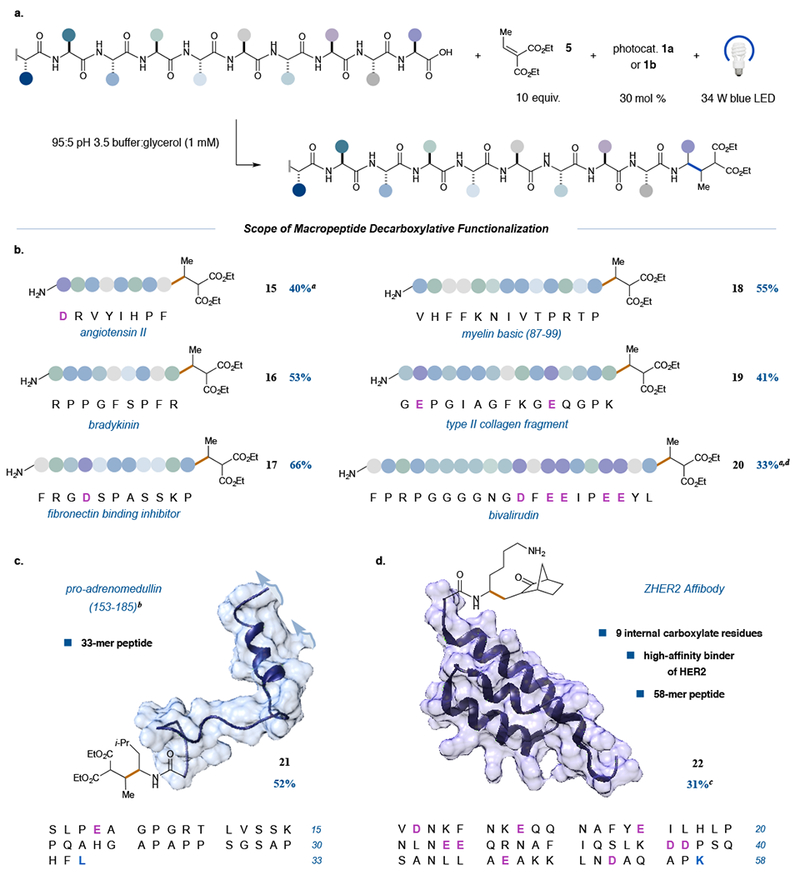
A variety of fully unprotected peptides from 8-mers to 58-mers can be site-selectively modified in this transformation, a, Generalized reaction scheme; b—d, substrate scope. All peptides are commercially available. In the case of the fibronectin binding inhibitor peptide (entry 17), the N-terminal Phe residue was added only for ease of analysis when initially developing the quantitative HPLC assay. Notably, longer peptides with nascent secondary structure (c) and peptides bearing high ratios of internal Asp and Glu residues (d) furnish C-terminal modified products exclusively. For entries 15–19 and 21–22, yields are reported as a % conversion as determined from reverse phase HPLC and are an average of three independent trials. aUsing photocatalyst lb. bRepresentative structure derived from the crystal structure of the full-length protein preproadrenomedullin. c3 equiv. photocatalyst lb and 3-methylene-2-norbomanone as the Michael acceptor. dYield reported as a combined % conversion after re-subjection of recovered starting material (see Supplementary Information).
