Figure 7.
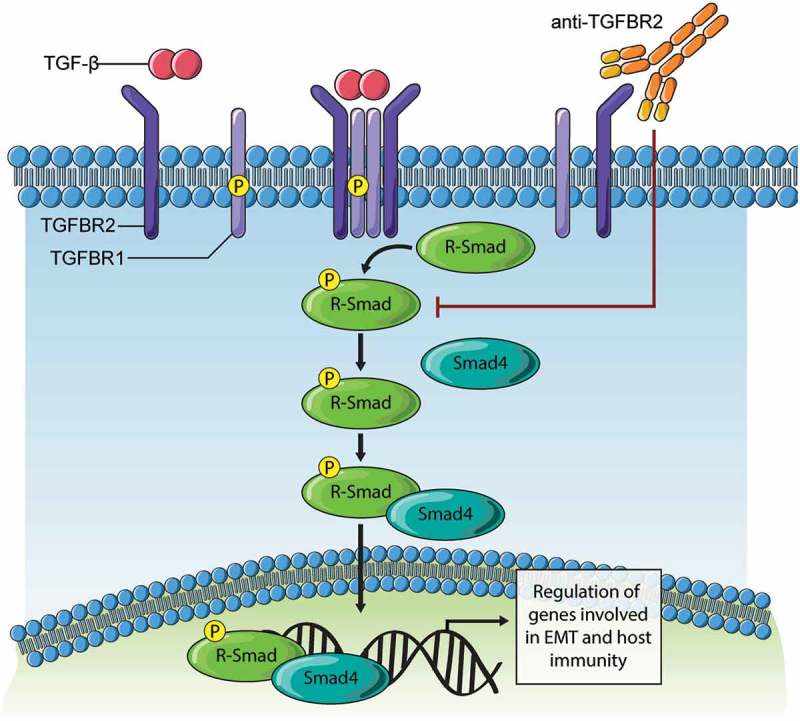
Model of TGFBR2 blockade effects on TGF-β signaling.
Binding of TGF-β ligand to TGFBR2 subunits results in clustering in a complex with TGFBR1/ALK5 that is competent for downstream signaling. The subsequent phosphorylation of receptor-activated SMAD (R-Smad) proteins (Smad2, Smad3) triggers interaction with Smad4 and nuclear entry. In tumor cells, this Smad complex promotes expression of genes driving EMT. In immune cells, this Smad complex promotes immune suppressive gene expression. The development of synthetic antibodies targeting TGFBR2 reported in this study, and their deployment in cancer models results in loss of TGF-β binding and downstream signaling. Thus, TGFBR2 blockade can evoke improved responses to chemotherapy in ovarian cancer models, and possibly other forms of therapy that rely on immune activation within the tumor microenvironment.
