Abstract
Background/Objectives
Nonpharmacological interventions such as biofeedback, cognitive behavioral therapy, and relaxation techniques are Level-A evidence-based treatments for headache. The impact of these interventions is often equivalent to or greater than pharmacological interventions, with fewer side effects. Despite such evidence, the rate of participation in nonpharmacological interventions for headache remains low. Once obstacles to optimizing use of behavioral interventions such as local access to nonpharmacological treatment and primary headache providers are traversed, identification of barriers contributing to low adherence is imperative given the high levels of disability and cost associated with treating headache disorders. In this review of factors in adults associated with underuse of nonpharmacological interventions, we discuss psychological factors relevant to participation in nonpharmacological treatment, including attitudes and beliefs, motivation for change, awareness of triggers, locus of control, self-efficacy, acceptance, coping styles, personality traits, and psychiatric comorbidities associated with treatment adherence. Finally, future prospects and approaches to optimizing treatment matching and minimizing adherence issues are addressed.
Methods
An interdisciplinary team conducted this narrative review. Neuropsychologists conducted a literature search during the month of July 2017 using a combination of the keywords (“headache” or “migraine”) and (“adherence” or “compliance”) or “barriers to treatment” or various “psychological factors” discussed in this narrative review. Content experts, a psychiatrist, and a complementary and integrative health specialist provided additional commentary and input to this narrative review resulting in integration of additional noteworthy studies, book chapters and books.
Results
Various psychological factors such as attitudes and beliefs, lack of motivation, poor awareness of triggers, external locus of control, poor self-efficacy, low levels of acceptance and engagement in maladaptive coping styles can contribute to non-adherence.
Conclusions
To maximize adherence, clinicians can assess and address an individual’s level of treatment acceptance, beliefs that may present as barriers, readiness for change, locus of control, self-efficacy and psychiatric comorbidities. Identification of barriers to adherence as well as the application of relevant assessment and intervention techniques have the potential to facilitate adherence and ultimately improve treatment success.
Keywords: Headache, Nonpharmacological interventions, Barriers to adherence, Psychological factors
INTRODUCTION
Although the efficacy of nonpharmacological interventions to treat headaches has been established,1 adherence rates are often low,2 creating challenges in management of headache as a chronic condition.3 While “compliance” typically refers to patients following recommendations from their health care providers,4 “adherence” has various definitions. Most originate from Haye’s definition, which is “the extent to which a person’s behavior coincides with medical or health advice.”5 For the purpose of this paper, we will use the word adherence, although the literature uses both terms, often interchangeably or without differentiation of their meaning.
Few studies have evaluated adherence to nonpharmacological headache treatment and the impact of adherence on clinical outcomes. A prospective cohort study of outpatients diagnosed with migraine and referred for behavioral treatment for migraine found that only about half (56.6%) of the patients initiated behavioral migraine treatment.6 In another study of 221 severe tertiary care headache inpatients who were advised to do relaxation therapy in the 7-day period before discharge, percentages of patients using relaxation on at least 5 of the 7 days were modest; 45% used relaxation during stress, 55% used relaxation during headache, and 59% used relaxation as a preventive measure.7 An observational study evaluating a 5-day multi-disciplinary headache treatment program involved training on lifestyle modification, progressive muscle relaxation (PMR) and physical therapy. After 12–18 months, 61% of patients were adherent to relaxation therapy (PMR) and 72% to aerobic endurance sports. Moreover, adherence to more than five lifestyle modifications was associated with at least a 50% reduction in headache frequency at follow up.8 In another study, although patient adherence to physician instructions across four areas - diet/meal timing, exercise, stress management/sleep modifications and medications/vitamins was low (14–45%),9 adherence in all four domains was associated with improvement in migraine disability, migraine days, and pain, as well as physician empathy.9
Despite the association between higher rates of adherence and better clinical outcomes, nonpharmacological treatment adherence remains varied and low. Identification of barriers to initiation and completion of nonpharmacological interventions is thus paramount to maximizing adherence and ultimately maximizing the potential effectiveness of these low risk interventions.10,11 This narrative review aims to identify barriers to poor adherence as a first step toward identifying potential targets to improve adherence.
METHODS
An interdisciplinary team conducted this narrative review of barriers to adherence to behavioral therapy for headache treatments. Four neuropsychologists (YM, DL, YSL, & FF) conducted literature searches in PubMed during the month of July 2017 using the following combination of descriptors: (“headache” or “migraine”), and (“adherence” or “compliance”) or “barriers to treatment” or the various psychological factors discussed in this narrative review. They synthesized the salient points relevant to nonpharmacological adherence and content experts (MTM, RL, & SP) each provided input and feedback leading to the addition of other pertinent studies. Subsequently, the full team (including a psychiatrist, NMS, and complementary and integrative health specialist, AS) added commentary resulting in integration of noteworthy studies. In addition to abstracts, book chapters and books addressing headaches and issues relevant to this narrative review were included. As this was not a systematic review with a PICO question, an a priori decision was made to examine studies with adherence as the primary outcome as well as other headache studies that examined barriers to following recommended behavioral interventions.
RESULTS
Barriers to Adherence
Attitudes and Beliefs
A major obstacle to treatment success, defined as 50% reduction in headache frequency, is the patient’s set of attitudes and beliefs regarding behavioral interventions.12 In turn, while studies examining the direct association between attitudes/beliefs and adherence are limited, existing studies of individuals with headache along with comorbid pain and insomnia have found that treatment acceptability was significantly associated with nonpharmacological treatment adherence.13 Therefore, perceptions of treatment acceptability may be a possible modifiable target to enhance adherence to nonpharmacological treatment adherence. Smitherman et al. provided a comprehensive list of attitudes and beliefs that patients have towards various nonpharmacological headache interventions and possible interventions to minimize barriers to adherence (Table 1).14
Table 1.
Potential problematic attitudes and beliefs towards nonpharmacological intervention and possible interventions14
| Nonpharmacological Interventions | Patient Attitudes and Beliefs | Possible Interventions |
|---|---|---|
| Relaxation Training | Patient upholding negative self-statements (e.g., I cannot relax and this technique won’t help me!”). | Use cognitive behavioral techniques (CBT) to help patient identify and modify these maladaptive/irrational thoughts. |
| Overly worried about his/her performance and upset that performance is below expectations. | Assist patient with establishing realistic goals. Indicate that he/she is trying too hard. May want to incorporate mental imagery or other mindfulness techniques to help patient to be in a more tranquil and “passive” state. | |
| Concern about losing control and being alert. | Discuss patient’s concerns about losing control. Provide education about how relaxation training can help patient proactively control/manage stress and headache. | |
| Biofeedback Training | Tries to make changes in biofeedback signals, but without success. | Encourage patient not to focus too much on the signal and output. Reassure patient that the training is a learning process and he/she will be able to do so gradually. |
| Perceives the task as a challenge rather than a tool to help him/her to relax. | Educate patient how a competitive attitude can be counterproductive. Encourage patient to adopt a more passive approach | |
| Concern and anxious about not being able to produce a desirable response on the signal. | Assess patient’s expectations. Provide reassurance and reinforcement that with practice, he/she will be able to obtain a realistic and desirable outcome. If patient experiences evaluation anxiety, consider allowing patient to complete the training without the presence of the therapist. | |
| Stress Management Training | Minimizes or unaware of the importance of this topic. | Provide education about how stress can exacerbate headache. |
| Lack of self-confidence in managing headache and stress. | Discuss the concept of locus of control and self-efficacy. Utilize CBT techniques to address any maladaptive beliefs of his/her ability. Empower patient to build-up confidence by practicing stress management techniques in between sessions and examine the actual and possible outcomes. Provide positive reinforcement. | |
| Trigger Management | Overwhelmed by the numbers of triggers. | Assist patient with prioritizing his/her triggers based on saliency. Also, ask patient to utilize a headache diary to explore patterns over time. |
| Lacks self-confidence to manage triggers. | Encourage patient to identify the triggers that are most amenable to changes and work on 1–2 triggers at a time. Provide positive reinforcement and ensure that patient documents and identifies his/her progress to increase self-confidence. Also, provide more structure to minimize failures. |
Used by permission from Headache by Todd A. Smitherman, Donald B. Penzien, Jeanetta C. Rains, Robert A. Nicholson, and Timothy T. Houle, ISBN 978–0-88937-328-0
©2015 Hogrefe Publishing www.hogrefe.com
Stage of Change Model
A patient’s readiness or motivation to change can influence adherence to treatment and is often represented via the Stage of Change Model (also known as the Transtheoretical Model),15 which identifies five stages – precontemplation, contemplation, preparation, action, and maintenance. Individuals in the pre-contemplation or contemplation stage may be less likely to adhere to treatment than individuals in the preparation, action, or maintenance stages as the former may be more ambivalent about following through with prescribed treatment (see Table 2 for a fuller description of these stages and ways of interacting with patients appropriate to their stage of change).
Table 2.
Stage of Change Model and Application to Headache Management
| Stage | Definition | Nicholson’s suggestion17 |
|---|---|---|
| Pre-contemplation | Patients not interested and do not acknowledge the need to make any changes to their behaviors. | Provide headache psychoeducation and increase patients’ awareness of the need and benefits to manage their headaches, such as keeping a diary and learning about their triggers. |
| Contemplation | Patients acknowledge that there is a problem, but are ambivalent about it. | Provide strategies to empower patients to manage their headaches (e.g., general medication management and learning to utilize their diary to manage their headaches). |
| Preparation | Patients have a desire to change and make a commitment to do so. | |
| Action | Patients take steps to proactively change. | Focus on problem-solving for specific challenges, such as acquiring ways to manage stressful situations or exploring which medications to take and when. |
| Maintenance | Patients maintain behavior changes. |
Lack of Knowledge or Unawareness of Headache Triggers
Treatment adherence can be affected by patients’ limited knowledge about or awareness of triggers that precede headaches, as failure to address these factors has been linked with suboptimal treatment outcomes.16 Educating patients about the nature of their headaches and helping them to identify headache onset patterns is a crucial component to managing their triggers. Headache triggers can be categorized into “avoidable” triggers such as alcohol, caffeine overuse or certain foods, odors, noises, or bright lights;14,17 “unavoidable and unmanageable” triggers such as hormonal changes and weather changes;14,16,17 and “unavoidable but manageable” lifestyle triggers such as stress, sleep, skipping meals, and medication non-adherence.17, 14 By learning to identify, monitor, prioritize, and manage the nature and triggers of their headaches, patients may gain success at better headache control, which in turn, may promote greater treatment adherence.14
Locus of Control
The term locus of control (LOC) refers to one’s perceived ability to influence life events.18 Internal LOC is defined as the perception that an event is entirely under one’s control, whereas external LOC refers to the perception that one has no ability to effect change on an event. Within the context of pain beliefs, external LOC is further divided into two dimensions: chance LOC and healthcare professional LOC.19 Chance LOC refers to the perception that events are due to luck or chance factors. By contrast, healthcare professional LOC is the belief that headache relief is primarily influenced by prescribed interventions from health care professionals. The Headache-Specific Locus of Control Scale, designed for recurrent headache sufferers, assesses the individual’s perceptions that headache problems and relief are determined by internal factors, health care professionals, or chance factors.20,21 Though links between LOC and headache outcomes across modalities of nonpharmacological treatment have long been established, to our knowledge, few studies have examined the relationship between adherence and LOC. Extant literature has generally demonstrated higher levels of treatment responsiveness among patients with internal LOC. For example, individuals with chronic headache who exhibited more than 50% pain reduction 6 months post autogenic and cognitive hypnosis training had higher pretreatment internal LOC relative to those classified as non-responders (i.e., with less than 50% pain reduction and early treatment termination).22 Similarly, higher internal LOC was associated with greater headache activity reduction 20 months following a biofeedback behavioral program.23 Moreover, Scharff et al.24 found that external LOC was associated with higher pain intensity,interference with various domains in their lives and adaptive responses. As such, one might surmise that the association between LOC and outcome is mediated by adherence. In a seminal study of 277 individuals with recurrent headaches, internal LOC was associated with a preference for self-regulation treatment and chance LOC was associated with higher levels of depression, physical symptoms, disability, and maladaptive coping strategies.20 Additionally, health care professional LOC was linked to preference for medical treatment and higher levels of medication use.
Taken together, evidence suggests patients with higher internal LOC may be more likely to adhere to nonpharmacological behavioral interventions with a self-management component. On the other hand, patients with high external LOC, particularly health care professional LOC may be more likely to adhere to pharmacological treatment. Thus, individual differences in LOC may be important to assess (see Table 3 for validated measures) in order to maximize adherence.
Table 3.
Measures
| Headache self-efficacy measures |
| Locus of Control Measures |
| Brief Psychiatric Inventories |
Self-Efficacy
Self-efficacy, the belief that one can successfully engage in a course of action to produce a desired outcome, is another psychological process long recognized to interact with headache treatment outcomes.25–27 Within the context of headache management, headache self-efficacy is defined as perceiving oneself capable of taking actions that will prevent or improve headache episodes.28,29 Though review of existing publications reveals a paucity of research directly examining associations between self-efficacy and nonpharmacological treatment adherence, available data indicate that greater levels of self-efficacy are associated with greater adherence to both preventative30 and acute pharmacological treatment.31 Further, self-efficacy mediated the association between pain severity and disability32,33 and may be an important mechanism in pain reduction.34 For example, French et al.35 found that self-efficacy scores were positively associated with use of adaptive coping efforts to both prevent and manage headache episodes among 146 individuals with diagnoses of tension-type headache or comorbid tension and migraine diagnoses. In contrast, low self-efficacy has been linked with less effective coping strategies and outcomes. Low self-efficacy was associated with use of passive coping strategies such as problem avoidance, wishful thinking, social withdrawal, and self-criticism in a sample of 153 college students with either tension or migraine headache.28 More recently, a systematic review found moderate evidence indicating that low self-efficacy was a potential prognostic indicator for poor outcome in treating chronic headache.36 These associations have led researchers to hypothesize that self-efficacy is critical for headache management, as lower self-efficacy may portend low adherence.37 Thus, assessing and targeting self-efficacy (see Table 3 for validated self-efficacy measures and Table 1 for strategies to facilitate self-efficacy) is recommended in order to facilitate adherence and responsiveness to nonpharmacological headache interventions.
Coping Styles
Individuals learn and utilize a variety of coping strategies to manage pain including relaxation, distraction, reappraisal, and seeking emotional support or spiritual comfort.38 According to Lazarus and Folkman, such coping strategies can be defined as either problem-focused (involves direct action to address the stressor) or emotion-focused (indirect attempts to attend to the psychological consequences of the stressor).39 Problem focused coping has been found to be effective for managing headaches, in that directly managing the stressful situation itself, generating solutions, and learning new skills to manage stressors reduces psychological distress and pain-related disability.40 While certain emotion-focused coping strategies such as problem avoidance, self-criticism, wishful thinking and social withdrawal are often associated with poorer outcomes,41 other emotion-focused coping styles such as emotional acceptance can be complementary to problem-focused coping.42 Overall, while a problem-focused coping style is compatible with a skills-based approach suggesting greater likelihood of nonpharmacological treatment adherence, emotion focused coping should be evaluated further.
When faced with chronic pain, the natural response may involve avoidance to control the pain. Avoidance, however, can have a paradoxical effect in that prolonged attempts to reduce aversive experiences may actually increase them.43 Further, unrealistic expectations of perfect headache control may escalate stress and hinder treatment adherence. In addition, there is a growing body of literature on anxiety sensitivity or the fear of arousal-related bodily sensations due to beliefs about presumed negative consequences.44 Anxiety sensitivity has been found to predict pain and adjustment to pain45 and has been considered a vulnerability factor for the engagement in fear and avoidance behaviors.46 Knowledge of patient beliefs about ability to control pain as well as anxiety sensitivity is useful in generating a specific treatment plan and is targeted in treatments such as acceptance and commitment therapy (ACT). In this treatment approach, acceptance is defined as “willingness to experience psychological states as they are.”47 More specifically, pain acceptance is conceptualized as having two components: 1) pain willingness, which is recognition that efforts to avoid or control pain are usually ineffective, and 2) activity engagement or the pursuit of life activities in a normal manner despite pain.48
Randomized controlled trials have shown that ACT is effective for chronic pain management.49 In one study, a one-session (5 hour) group ACT workshop and headache education was compared to treatment as usual (TAU) for patients with migraine and comorbid depression.50 At 12-week follow up, ACT participants demonstrated significantly greater reductions in both depressive symptoms and headache-related disability.50 In a separate publication reporting the effects of an ACT plus Migraine Education (ACT-ED) workshop versus treatment as usual (TAU) on headache, the ACT-ED group demonstrated significantly greater reductions than the TAU group in headache frequency, headache severity, and use of acute medications for headache at 3-month follow up.51 Other studies have found that individuals with lower levels of pain acceptance report greater pain-related interference, catastrophizing, and disengagement from activities52 as well as depression and disability.53
Theoretical considerations and extant evidence from acceptance-based interventions suggest that adherence may be improved by targeting an individual’s beliefs and behavior related to pain avoidance and control. In turn, adherence to acceptance-based interventions may be improved by changing the focus from perfect pain control to improving function by facilitating engagement in activities despite pain.
Psychiatric Comorbidities
Adherence to nonpharmacological headache treatment recommendations may be hindered by comorbid psychiatric conditions. For example, meta-analyses of anxiety disorders have reported non-adherence in the range of 9–21% for cognitive behavioral therapy (CBT: across meta-analyses, M = 16%).54 Studies examining CBT for depression and anxiety have also revealed non-adherence rates of approximately 20–30%.55,56 Thus, individuals who require treatment for comorbid psychiatric conditions face multiple psychosocial factors that complicate adherence. As such, addressing comorbidities such as anxiety or depression, which may contribute to avoidance behaviors or impact motivation may be helpful. Therefore, given the diversity of the psychiatric comorbidities with migraine and the differing treatment considerations, one needs to take into account psychiatric comorbidities when considering adherence for nonpharmacological treatment for migraine (see Table 3 for screening measures).
Enhancing Adherence
We have set forth several points above regarding psychological aspects, which can present as major obstacles to nonpharmacological treatment adherence. The following are some potential evidence based and theoretical ways in which adherence might be facilitated.
Address Ambivalence to Treatment
Certain attitudes and beliefs towards headache nonpharmacological treatment can be assessed in order to increase adherence once treatment is initiated. Smitherman et al. have provided a comprehensive list of patient attitudes and beliefs towards various headache nonpharmacological interventions and possible strategies clinicians can utilize to address various concerns (See Table 1).14 Nicholson outlined the importance of the clinician’s ability to understand and shape headache nonpharmacological treatment based on the patient’s readiness to change.17 Table 2 summarizes strategies that healthcare providers can implement that correspond to patients’ various stages of readiness to change during headache nonpharmacological treatment.
Motivational Interviewing
One way to promote change among patients is to utilize Motivational Interviewing (MI), an evidence-based intervention57 that focuses on clinician and patient collaboration to cultivate changes without the clinician imposing judgment. This intervention effectively promotes self-efficacy, coping with resistance, and exploration of any ambivalence associated with headache management. Strategies for MI include using open-ended questions to elicit self-motivational statements and behavioral change from the client in addition to creating client discrepancy between behaviors and statements to enhance motivation for positive change.57 Furthermore, patients’ strengths to increase self-efficacy are affirmed.57
Treatment Matching
A more formal potential approach to increasing adherence is treatment matching, which tailors treatment to the patient characteristics, preferences, symptoms, motivation, readiness to change, and level of severity.58 While these factors are important to consider when referring patients for nonpharmacological headache interventions, limited algorithms have been created to match interventions with headache patients’ profiles.58 Treatment matching has been utilized more in other populations, such as individuals with substance abuse. Although no matching criteria have been developed for the headache population, Lipchik et al. suggested two techniques to study headache treatment matching: patient typologies and Aptitude Treatment Interaction (ATI).58
The patient typologies methodology uses statistical analysis to identify clusters of patient profiles, the underlying assumption being that each patient’s profile predicts different treatment needs and the approach that should result in the greatest treatment gains.58 For instance, headache patients can be grouped into five clusters: i) “just painful” patients (those without significant behavioral disturbances), ii) anxious, depressed, and mildly-moderately affected patients, iii) patients misusing medications, iv) patients overusing headache medications, and v) patients with personality disorder(s).59 The need for treatment matching with patient typologies is supported by the fact that ineffective interventions can negatively affect treatment adherence.60 For example, a directive approach with cognitive behavioral therapy can be counterintuitive for patients with narcissistic or borderline personality disorder.61 Drawbacks are that patient typologies are not informed by actual patient response data and it can also be problematic when patients do not clearly fall under any of or more than one of the clusters.62 Further, various psychological factors noted previously (e.g., locus of control, self-efficacy, etc.) are not considered in typologies.
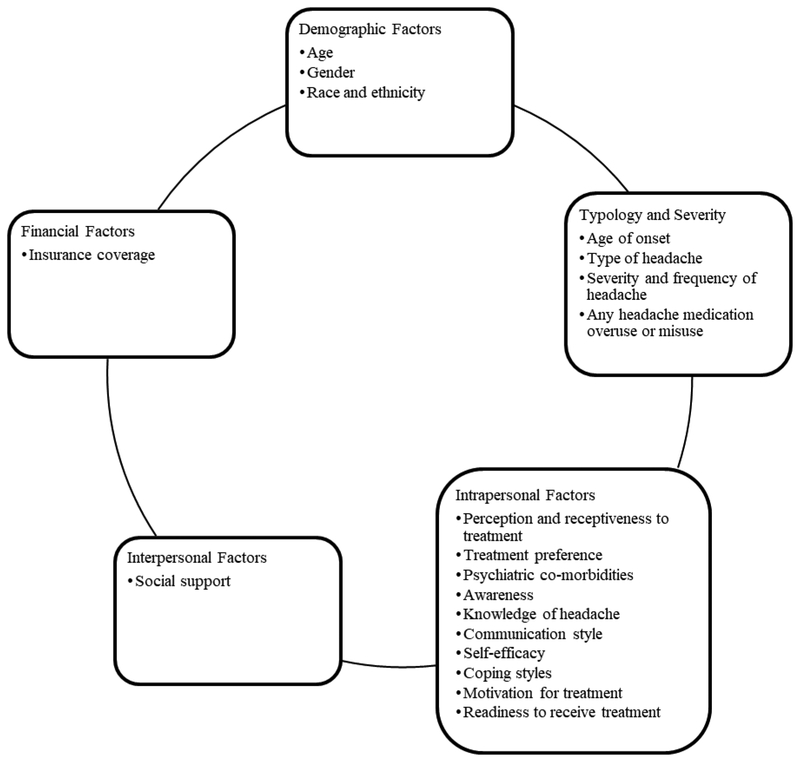
ATI methodology focuses on predicting treatment outcomes based on a critical aptitude or characteristic. Common patient aptitudes examined include 1) patient demographic variables, 2) headache severity, chronicity, type, and disability, 3) psychiatric co-morbidities, and 4) psychological factors, such as self-esteem, coping styles, and motivation for treatment. Typically, individuals with more of a certain aptitude or characteristic would benefit and adhere to a particular treatment, while individuals with less would potentially benefit from a different type of treatment. One drawback of this approach is that if too many variables are analyzed and the statistical power is poor, results will not be interpretable.58 Thus, it is crucial for researchers and clinicians to conduct a power analysis to identify therapy-specific predictors that are strong in theoretical validity.58 Figure 1 provides a sample of factors that can be considered for ATI analysis.
Figure 1.
Potential factors that can be considered for Aptitude-Treatment Interaction (ATI) for headache treatment interventions.
Utilization of Psychiatric/Personality Assessment Tools
Evaluation of personality traits and psychiatric symptoms with objective standardized assessment tools can identify comorbid psychiatric disorders that may contribute to headache chronicity.63–65 As noted prior, individuals who require treatment for comorbid psychiatric conditions face multiple psychosocial factors that complicate adherence, which suggests the importance of assessing comorbidities. While the MMPI is a well validated and commonly used measure to assess personality traits, studies examining the utility of the MMPI among individuals with headache to predict adherence are limited. Of existing studies utilizing the MMPI, many have examined the prognostic role for pretreatment MMPI scores in treatment outcome rather than adherence.66,67 A more recent study with a longer follow up than prior studies found that the group who showed 50% reduction in headache two years after treatment had significantly lower baseline MMPI-2 scores on scales indicative of excessive somatic focus (i.e., elevations on scales 1, 2, 3) an non-reality based thoughts compared to treatment non-responders.68 While there is also a growing body of literature examining personality traits associated with adherence for medication overuse headache detoxification,69–71 studies on personality and nonpharmacaological headache treatment adherence are scarce. One study examined utilization of the MMPI as a prognostic indicator of adherence and found that individuals with either tension or migraine headache who had higher scores in scales characterized by anti-social, paranoid, or manic tendencies were more likely to terminate biofeedback and relaxation training prematurely.72
Utilization of the MMPI may not be practical in most clinical settings given the amount of time required to administer, score, and interpret the measure. Therefore, screeners such as Generalized Anxiety Disorder – 7 item (GAD)73 or the Patient Health Questionnaire – 9 (PHQ-9)74 may be a valuable alternative to assess psychiatric comorbidities. Altogether, these findings suggest that examining personality traits and characteristics with measures such as the MMPI or other validated measures of personality traits and psychiatric symptoms (Table 3) has some clinical utility in choosing appropriate treatment for headache populations, with relevance for adherence and treatment responsiveness.
Conclusions
Despite a growing body of literature demonstrating positive outcomes among individuals who adhere to nonpharmacological treatment, adherence to such treatments remains an issue. Indeed, initial considerations include obstacles that contribute to under-utilization of nonpharmacological interventions. Such factors include limited availability of primary headache providers offering appropriate interventions or referrals as well as health care providers trained in nonpharmacological treatment. Once above factors are addressed, adherence becomes an issue. The focus of this narrative review was to examine ways in which various psychological factors and psychiatric comorbidities may compromise treatment adherence. Furthermore, potential methods to enhance treatment adherence were discussed.
Various psychological factors such as attitudes or beliefs, lack of motivation, poor awareness of triggers, external locus of control, poor self-efficacy, low levels of pain acceptance, and engagement in maladaptive coping styles can all contribute to non-adherence. To maximize adherence, clinicians can begin by assessing an individual’s perception of nonpharmacological treatment acceptance by initiating a dialogue and asking for patient’s thoughts or reactions regarding such interventions. Any problematic attitudes and beliefs that may present as barriers should be addressed by providing education, encouragement, and by managing expectations, etc. (see Table 1 for possible interventions). Further, clinicians can assess readiness for change and utilize motivational interviewing techniques for individuals in the pre-contemplation or contemplation stage including providing headache psychoeducation to assist in increasing awareness of the benefits of managing their headaches (see Table 2). Additionally, brief measures to assess locus of control and self-efficacy can be utilized to guide ideal treatment approach (see Table 3). Further, given psychiatric comorbidities that are associated with headache can impact treatment non-adherence,54,75 a comprehensive assessment and history-taking is of utmost importance (see Table 3).
Developing personalized treatment approach based on assessment of the above psychological and psychiatric factors and utilizing treatment matching approaches such as those proposed by Lipchik et al.58 may maximize adherence. For example, understanding that individuals with higher internal locus of control may be more receptive to nonpharmacological intervention as opposed to individuals with external locus of control whose preference may be for pharmacological treatment, can facilitate successful treatment allocation. Furthermore, given theoretical considerations and extant evidence that adaptive coping may promote adherence to nonpharmacological interventions for headache, evidence-based interventions targeting dysfunctional coping skills may promote treatment adherence. As an example, combining problem-focused and adaptive emotional coping skills such as pain acceptance may be potentially beneficial.42 The inverse relationship between levels of pain acceptance and interference in daily activities,52,53 supports the importance of efforts to increase pain acceptance by implementing flexible behavioral responses to pain, rather than focusing on pain reduction.
While growing, adherence research for nonpharmacological headache treatment remains scant. Understanding and addressing factors associated with adherence is paramount to improving clinical outcome. Future studies might utilize treatment matching algorithms by considering psychological and psychiatric factors in order to assess adherence rates with a more personalized approach. For example, based on patient typologies and ATI as discussed by Lipchik et al. (2005) creating a patient-treatment matching protocol consisting of various intervention modules (e.g., CBT, medication management, trigger management, motivational interviewing, acceptance and commitment therapy, etc.) may be a more tailored and tolerated approach to treatment. Attention to the variety of factors that influence adherence is paramount to headache practitionres in promoting treatment adherence and ultimately treatment outcome.
Acknowledgments
Funding: No funding. Dr. Minen is a recipient of the AAN-ABF Practice Research Training Fellowship, the ECRIP grant, and a NIH K23 grant (AT009706–01) which funds her time to conduct research.
Footnotes
Financial Disclosures:
Dr. Yuka Matsuzawa: no conflicts of interest.
Dr. Yuen Shan Christine Lee: no conflicts of interest.
Dr. Felicia Fraser: no conflicts of interest.
Dr. Donna Langenbahn: no conflicts of interest
Dr. Amanda Shallcross: no conflicts of interest
Dr. Scott Powers- No conflict
Dr. Richard Lipton- Dr. Richard B. Lipton receives support from the Migraine Research Foundation and the National Headache Foundation. He holds stock options in eNeura Therapeutics; serves as consultant, advisory board member, or has received honoraria from: Alder, Allergan, American Headache Society, Amgen, Autonomic Technologies, Avanir, Boston Scientific, Colucid, Dr. Reddy’s, Electrocore, Eli Lilly, eNeura Therapeutics, Glaxo, Inc., Merck, GlaxoSmithKlein (formerly Novartis), Pfizer, Teva, Vedanta. He receives royalties from Wolff’s Headache, 8th Edition, Oxford Press University, 2009 and Informa.
Dr. Naomi Simon-No conflict
Dr. Mia Minen: Dr. Minen is a recipient of the AAn-ABF Practice Research Training Fellowship, the ECRIP grant, and a NIH K23 grant (AT009706–01) which pays for time to conduct research.
Contributor Information
Yuka Matsuzawa, Department of Rehabilitation Medicine, NYU Langone Health
Yuen Shan Christine Lee, Department of Rehabilitation Medicine, NYU Langone Health.
Felicia Fraser, Department of Physical Medicine & Rehabilitation, the MetroHealth System.
Donna Langenbahn, Department of Rehabilitation Medicine, NYU Langone Health.
Amanda Shallcross, Department of Population Health, NYU Langone Health
Scott Powers, Division of Behavioral Medicine & Clinical Psychology, Cincinnati Children’s Hospital
Richard Lipton, Department of Neurology, Albert Einstein College of Medicine.
Naomi Simon, Department of Psychiatry, NYU Langone Medical Center.
Mia Minen, Department of Neurology, NYU Langone Medical Center).
References
- 1.Rains JC, Penzien DB, McCrory DC, Gray RN. Behavioral headache treatment: History, review of the empirical literature, and methodological critique. Headache. 2005;45 Suppl 2:S92–109. doi: HED4502003 [pii]. [DOI] [PubMed] [Google Scholar]
- 2.Ramsey RR, Ryan JL, Hershey AD, Powers SW, Aylward BS, Hommel KA. Treatment adherence in patients with headache: A systematic review. Headache. 2014;54(5):795–816. doi: 10.1111/head.12353 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dunbar-Jacob J, Mortimer-Stephens MK. Treatment adherence in chronic disease. J Clin Epidemiol. 2001;54 Suppl 1:S57–60. doi: S0895435601004577 [pii]. [DOI] [PubMed] [Google Scholar]
- 4.Urquhart J Patient non-compliance with drug regimens: Measurement, clinical correlates, economic impact. Eur Heart J. 1996;17 Suppl A:8–15. [DOI] [PubMed] [Google Scholar]
- 5.Rains JC, Penzien DB, Lipchik GL. Behavioral facilitation of medical treatment of headache: Implications of noncompliance and strategies for improving adherence. Headache. 2006;46 Suppl 3:S142–3. doi: HED565 [pii]. [DOI] [PubMed] [Google Scholar]
- 6.Minen MT, Azarchi S, Sobolev R, et al. Factors related to migraine patients’ decisions to follow a headache specialist’s recommendation for migraine behavioral treatment: A prospective observational study. To be presented at the AAN annual meeting 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoodin F, Brines BJ, Lake AE 3rd, Wilson J, Saper JR. Behavioral self-management in an inpatient headache treatment unit: Increasing adherence and relationship to changes in affective distress. Headache. 2000;40(5):377–383. doi: hed57 [pii]. [DOI] [PubMed] [Google Scholar]
- 8.Gaul C, van Doorn C, Webering N, et al. Clinical outcome of a headache-specific multidisciplinary treatment program and adherence to treatment recommendations in a tertiary headache center: An observational study. J Headache Pain. 2011;12(4):475–483. doi: 10.1007/s10194-011-0348-y [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Attar HS, Chandramani S. Impact of physician empathy on migraine disability and migraineur compliance. Ann Indian Acad Neurol. 2012;15(Suppl 1):S89–94. doi: 10.4103/0972-2327.100025 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campbell J, Penzien D, Wall E. Evidence-based guidelines for migraine headache: Behavioral and physical treatments. . 2000.
- 11.Kropp P, Meyer B, Dresler T, et al. Relaxation techniques and behavioural therapy for the treatment of migraine : Guidelines from the german migraine and headache society. Schmerz. 2017;31(5):433–447. doi: 10.1007/s00482-017-0214-1 [doi]. [DOI] [PubMed] [Google Scholar]
- 12.Holroyd KA, Penzien DB, Lipchik GL. Behavioral management of headache In: Silberstein SD, Lipton RB, Dalessio DJ, eds. Wolff’s headache and other head pain. 7th ed. New York: Oxford University Press; 2001:562. [Google Scholar]
- 13.Koffel E, Vitiello MV, McCurry SM, Rybarczyk B, Von Korff M. Predictors of adherence to psychological treatment for insomnia and pain: Analysis from a randomized trial. Clin J Pain. 2018;34(4):375–382. doi: 10.1097/AJP.0000000000000546 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smitherman TA, Penzien DB, Rains JC, Nicholson RA, Houle TT. Headache. Boston, MA: Hogrefe Publishing; 2015. [Google Scholar]
- 15.Prochaska JO, DiClemente CC. Self change processes, self efficacy and decisional balance across five stages of smoking cessation. Prog Clin Biol Res. 1984;156:131–140. [PubMed] [Google Scholar]
- 16.Lipton RB, Silberstein SD, Saper JR, Bigal ME, Goadsby PJ. Why headache treatment fails. Neurology. 2003;60(7):1064–1070. [DOI] [PubMed] [Google Scholar]
- 17.Nicholson RA. Chronic headache: The role of the psychologist. Curr Pain Headache Rep. 2010;14(1):47–54. doi: 10.1007/s11916-009-0087-9 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rotter JB. Generalized expectancies for internal versus external control of reinforcement. Psychol Monogr. 1966;80(1):1–28. [PubMed] [Google Scholar]
- 19.Wallston KA, Wallston BS, DeVellis R. Development of the multidimensional health locus of control (MHLC) scales. Health Educ Monogr. 1978;6(2):160–170. [DOI] [PubMed] [Google Scholar]
- 20.Martin NJ, Holroyd KA, Penzien DB. The headache-specific locus of control scale: Adaptation to recurrent headaches. Headache. 1990;30(11):729–734. [DOI] [PubMed] [Google Scholar]
- 21.Grinberg AS, Seng EK. Headache-specific locus of control and migraine-related quality of life: Understanding the role of anxiety. Int J Behav Med. 2017;24(1):136–143. doi: 10.1007/s12529-016-9587-2 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.ter Kuile MM, Spinhoven P, Linssen AC. Responders and nonresponders to autogenic training and cognitive self-hypnosis: Prediction of short- and long-term success in tension-type headache patients. Headache. 1995;35(10):630–636. [DOI] [PubMed] [Google Scholar]
- 23.Hudzinski LG, Levenson H. Biofeedback behavioral treatment of headache with locus of control pain analysis: A 20-month retrospective study. Headache. 1985;25(7):380–386. [DOI] [PubMed] [Google Scholar]
- 24.Scharff L, Turk DC, Marcus DA. The relationship of locus of control and psychosocial-behavioral response in chronic headache. Headache. 1995;35(9):527–533. [DOI] [PubMed] [Google Scholar]
- 25.Holroyd KA, Penzien DB, Hursey KG, et al. Change mechanisms in EMG biofeedback training: Cognitive changes underlying improvements in tension headache. J Consult Clin Psychol. 1984;52(6):1039–1053. [DOI] [PubMed] [Google Scholar]
- 26.Bandura A, O’Leary A, Taylor CB, Gauthier J, Gossard D. Perceived self-efficacy and pain control: Opioid and nonopioid mechanisms. J Pers Soc Psychol. 1987;53(3):563–571. [DOI] [PubMed] [Google Scholar]
- 27.Nicholson RA, Houle TT, Rhudy JL, Norton PJ. Psychological risk factors in headache. Headache. 2007;47(3):413–426. doi: HED716 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin NJ, Holroyd KA, Rokicki LA. The headache self-efficacy scale: Adaptation to recurrent headaches. Headache. 1993;33(5):244–248. [DOI] [PubMed] [Google Scholar]
- 29.Turner DP, Houle TT. Psychological evaluation of a primary headache patient. Pain Manag. 2013;3(1):19–25. doi: 10.2217/pmt.12.77 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heckman BD, Ellis G. Preventive medication adherence in african american and caucasian headache patients. Headache. 2011;51(4):520–532. doi: 10.1111/j.1526-4610.2011.01866.x [doi]. [DOI] [PubMed] [Google Scholar]
- 31.Seng EK, Holroyd KA. Optimal use of acute headache medication: A qualitative examination of behaviors and barriers to their performance. Headache. 2013;53(9):1438–1450. [DOI] [PubMed] [Google Scholar]
- 32.Graef JE, Rief W, French DJ, Nilges P, Nestoriuc Y. German language adaptation of the headache management self-efficacy scale (HMSE-G) and development of a new short form (HMSE-G-SF). Headache. 2015;55(7):958–972. doi: 10.1111/head.12564 [doi]. [DOI] [PubMed] [Google Scholar]
- 33.Peck KR, Smitherman TA. Mediator variables in headache research: Methodological critique and exemplar using self-efficacy as a mediator of the relationship between headache severity and disability. Headache. 2015;55(8):1102–1111. doi: 10.1111/head.12633 [doi]. [DOI] [PubMed] [Google Scholar]
- 34.Stonnington CM, Kothari DJ, Davis MC. Understanding and promoting resiliency in patients with chronic headache. Curr Neurol Neurosci Rep. 2016;16(1):6–015–0609–2. doi: 10.1007/s11910-015-0609-2 [doi]. [DOI] [PubMed] [Google Scholar]
- 35.French DJ, Holroyd KA, Pinell C, Malinoski PT, O’Donnell F, Hill KR. Perceived self-efficacy and headache-related disability. Headache. 2000;40(8):647–656. doi: hed00108 [pii]. [DOI] [PubMed] [Google Scholar]
- 36.Probyn K, Bowers H, Caldwell F, et al. Prognostic factors for chronic headache: A systematic review. Neurology. 2017;89(3):291–301. doi: 10.1212/WNL.0000000000004112 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rains JC, Penzien DB, Lipchik GL. Behavioral facilitation of medical treatment of headache: Implications of noncompliance and strategies for improving adherence. . 2006;46 Suppl 3:S142–3. [DOI] [PubMed] [Google Scholar]
- 38.Keefe FJ, Affleck G, Lefebvre JC, Starr K, Caldwell DS, Tennen H. Pain coping strategies and coping efficacy in rheumatoid arthritis: A daily process analysis. Pain. 1997;69(1–2):35–42. doi: S0304–3959(96)03246–0 [pii]. [DOI] [PubMed] [Google Scholar]
- 39.Lazarus R, Folkman S. Stress, appraisal, and coping. New York: Springer Publishing Company; 1984. [Google Scholar]
- 40.Keefe FJ, Rumble ME, Scipio CD, Giordano LA, Perri LM. Psychological aspects of persistent pain: Current state of the science. J Pain. 2004;5(4):195–211. doi: 10.1016/j.jpain.2004.02.576 [doi]. [DOI] [PubMed] [Google Scholar]
- 41.Hassinger HJ, Semenchuk EM, O’Brien WH. Appraisal and coping responses to pain and stress in migraine headache sufferers. J Behav Med. 1999;22(4):327–340. [DOI] [PubMed] [Google Scholar]
- 42.Shallcross AJ, Troy A, Mauss IB. Regulation of emotions under stress In: Scott R, Kosslyn S, eds. Emerging trends in the social and behavioral sciences. ; 2015. [Google Scholar]
- 43.Wenzlaff RM, Wegner DM. Thought suppression. Annu Rev Psychol. 2000;51:59–91. doi: 10.1146/annurev.psych.51.1.59 [doi]. [DOI] [PubMed] [Google Scholar]
- 44.Taylor S, Zvolensky MJ, Cox BJ, et al. Robust dimensions of anxiety sensitivity: Development and initial validation of the anxiety sensitivity index-3. Psychol Assess. 2007;19(2):176–188. doi: 2007-07953-002 [pii]. [DOI] [PubMed] [Google Scholar]
- 45.Smitherman TA, Davis RE, Walters AB, Young J, Houle TT. Anxiety sensitivity and headache: Diagnostic differences, impact, and relations with perceived headache triggers. Cephalalgia. 2015;35(8):710–721. doi: 10.1177/0333102414557840 [doi]. [DOI] [PubMed] [Google Scholar]
- 46.Ocanez KL, McHugh RK, Otto MW. A meta-analytic review of the association between anxiety sensitivity and pain. Depress Anxiety. 2010;27(8):760–767. doi: 10.1002/da.20681 [doi]. [DOI] [PubMed] [Google Scholar]
- 47.Thompson M, McCracken LM. Acceptance and related processes in adjustment to chronic pain. Curr Pain Headache Rep. 2011;15(2):144–151. doi: 10.1007/s11916-010-0170-2 [doi]. [DOI] [PubMed] [Google Scholar]
- 48.Buse DC, Silberstein SD, Manack AN, Papapetropoulos S, Lipton RB. Psychiatric comorbidities of episodic and chronic migraine. J Neurol. 2013;260(8):1960–1969. doi: 10.1007/s00415-012-6725-x [doi]. [DOI] [PubMed] [Google Scholar]
- 49.McCracken LM, Vowles KE. Acceptance and commitment therapy and mindfulness for chronic pain: Model, process, and progress. Am Psychol. 2014;69(2):178–187. doi: 10.1037/a0035623 [doi]. [DOI] [PubMed] [Google Scholar]
- 50.Dindo L, Recober A, Marchman JN, Turvey C, O’Hara MW. One-day behavioral treatment for patients with comorbid depression and migraine: A pilot study. Behav Res Ther. 2012;50(9):537–543. doi: 10.1016/j.brat.2012.05.007 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dindo L, Recober A, Marchman J, O’Hara MW, Turvey C. One-day behavioral intervention in depressed migraine patients: Effects on headache. Headache. 2014;54(3):528–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chiros C, O’Brien WH. Acceptance, appraisals, and coping in relation to migraine headache: An evaluation of interrelationships using daily diary methods. J Behav Med. 2011;34(4):307–320. doi: 10.1007/s10865-011-9313-0 [doi]. [DOI] [PubMed] [Google Scholar]
- 53.Dindo L, Recober A, Marchman J, O’Hara M, Turvey C. Depression and disability in migraine: The role of pain acceptance and values-based action. Int J Behav Med. 2015;22(1):109–117. doi: 10.1007/s12529-014-9390-x [doi]. [DOI] [PubMed] [Google Scholar]
- 54.Olatunji BO, Cisler JM, Tolin DF. A meta-analysis of the influence of comorbidity on treatment outcome in the anxiety disorders. Clin Psychol Rev. 2010;30(6):642–654. doi: 10.1016/j.cpr.2010.04.008 [doi]. [DOI] [PubMed] [Google Scholar]
- 55.Williams C, Wilson P, Morrison J, et al. Guided self-help cognitive behavioural therapy for depression in primary care: A randomised controlled trial. PLoS One. 2013;8(1):e52735. doi: 10.1371/journal.pone.0052735 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Luik AI, Bostock S, Chisnall L, et al. Treating depression and anxiety with digital cognitive behavioural therapy for insomnia: A real world NHS evaluation using standardized outcome measures. Behav Cogn Psychother. 2017;45(1):91–96. doi: S1352465816000369 [pii]. [DOI] [PubMed] [Google Scholar]
- 57.Miller WR & Rollnick S Motivational interviewing helping people change. Third ed. New York: The Guilford Press; 2012. [Google Scholar]
- 58.Lipchik GL, Nicholson RA, Penzien DB. Allocation of patients to conditions in headache clinical trials: Randomization, stratification, and treatment matching. Headache. 2005;45(5):419–428. doi: HED05093 [pii]. [DOI] [PubMed] [Google Scholar]
- 59.Saper JR. “Are you talking to me?” confronting behavioral disturbances in patients with headache. Headache. 2006;46 Suppl 3:S151–6. doi: HED569 [pii]. [DOI] [PubMed] [Google Scholar]
- 60.Rafii F, Fatemi NS, Danielson E, Johansson CM, Modanloo M. Compliance to treatment in patients with chronic illness: A concept exploration. Iran J Nurs Midwifery Res. 2014;19(2):159–167. [PMC free article] [PubMed] [Google Scholar]
- 61.Cukrowicz KC&JJ TE. Treating the “Mischances of character,” simply and effectively. J Contemp Psychother. 2005;35(2):157. [Google Scholar]
- 62.Snow RE. Aptitude-treatment interaction as a framework for research on individual differences in psychotherapy. J Consult Clin Psychol. 1991;59(2):205–216. [DOI] [PubMed] [Google Scholar]
- 63.Breslau N, Andreski P. Migraine, personality, and psychiatric comorbidity. Headache. 1995;35(7):382–386. [DOI] [PubMed] [Google Scholar]
- 64.Smitherman TA, Penzien DB, Rains JC. Challenges of nonpharmacologic interventions in chronic tension-type headache. Curr Pain Headache Rep. 2007;11(6):471–477. [DOI] [PubMed] [Google Scholar]
- 65.Tan HJ, Suganthi C, Dhachayani S, Rizal AM, Raymond AA. The coexistence of anxiety and depressive personality traits in migraine. Singapore Med J. 2007;48(4):307–310. [PubMed] [Google Scholar]
- 66.Werder DS, Sargent JD, Coyne L. MMPI profiles of headache patients using self-regulation to control headache activity. Headache. 1981;21(4):164–169. [DOI] [PubMed] [Google Scholar]
- 67.Mongini F, Ibertis F, Ferla E. Personality characteristics before and after treatment of different head pain syndromes. Cephalalgia. 1994;14(5):368–73; discussion 319. doi: 10.1046/j.1468-2982.1994.1405368.x [doi]. [DOI] [PubMed] [Google Scholar]
- 68.Luconi R, Bartolini M, Taffi R, et al. Prognostic significance of personality profiles in patients with chronic migraine. Headache. 2007;47(8):1118–1124. doi: HED807 [pii]. [DOI] [PubMed] [Google Scholar]
- 69.Sances G, Galli F, Ghiotto N, et al. Factors associated with a negative outcome of medication-overuse headache: A 3-year follow-up (the ‘CARE’ protocol). Cephalalgia. 2013;33(7):431–443. doi: 10.1177/0333102413477737 [doi]. [DOI] [PubMed] [Google Scholar]
- 70.Bottiroli S, Viana M, Sances G, et al. Psychological factors associated with failure of detoxification treatment in chronic headache associated with medication overuse. Cephalalgia. 2016;36(14):1356–1365. doi: 0333102416631960 [pii]. [DOI] [PubMed] [Google Scholar]
- 71.Corbelli I, Caproni S, Eusebi P, Sarchielli P. Drug-dependence behaviour and outcome of medication-overuse headache after treatment. J Headache Pain. 2012;13(8):653–660. doi: 10.1007/s10194-012-0492-z [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tsushima WT, Stoddard VM, Tsushima VG, Daly J. Characteristics of treatment drop-outs among two samples of chronic headache patients. J Clin Psychol. 1991;47(2):199–205. [DOI] [PubMed] [Google Scholar]
- 73.Spitzer RL, Kroenke K, Williams JB, Lowe B. A brief measure for assessing generalized anxiety disorder: The GAD-7. Arch Intern Med. 2006;166(10):1092–1097. doi: 166/10/1092 [pii]. [DOI] [PubMed] [Google Scholar]
- 74.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: jgi01114 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Taylor S, Abramowitz JS, McKay D. Non-adherence and non-response in the treatment of anxiety disorders. J Anxiety Disord. 2012;26(5):583–589. doi: 10.1016/j.janxdis.2012.02.010 [doi]. [DOI] [PubMed] [Google Scholar]
- 76.Nicholas MK. The pain self-efficacy questionnaire: Taking pain into account. Eur J Pain. 2007;11(2):153–163. doi: S1090–3801(05)00193-X [pii]. [DOI] [PubMed] [Google Scholar]
- 77.Schwarzer R, Jerusalem M. Generalized self-efficacy scale In: Measures in health psychology: A user’s portfolio. casual and control beliefs. Windsor, UK: NFER-NELSON; 1995:35. [Google Scholar]
- 78.Wallston KA, Wallston BS, DeVellis R. Development of the multidimensional health locus of control (MHLC) scales. Health Educ Monogr. 1978;6(2):160–170. [DOI] [PubMed] [Google Scholar]
- 79.Penzien DB, Moseley T, Knowlton G, Slipan C, Holm JE, Curtis K. Psychometric characteristics of the pain locus of control scale. Proc Am Pain Soc. 1989;8(68). [Google Scholar]
- 80.ter Kuile MM, Linssen A, Spinhoven P. The development of the multidimensional locus of pain control questionnaire (MLPC): Factor structure, reliability, and validity. J Psychopathol Behav Assess. 1993;15:387–404. [Google Scholar]
- 81.Martin NJ, Holroyd KA, Penzien DB. The headache-specific locus of control scale: Adaptation to recurrent headaches. Headache. 1990;30(11):729–734. [DOI] [PubMed] [Google Scholar]
- 82.Derogatis LR, Melisaratos N. The brief symptom inventory: An introductory report. Psychol Med. 1983;13(3):595–605. [PubMed] [Google Scholar]
- 83.Overall JE, Gorham DR. The brief psychiatric rating scale. Psychological reports. 1962;10:799–812. [Google Scholar]
- 84.Derogotis LR. SCL-90-R administration, scoring, and procedures manual. National Computer System; 1994. [Google Scholar]