Figure 6.
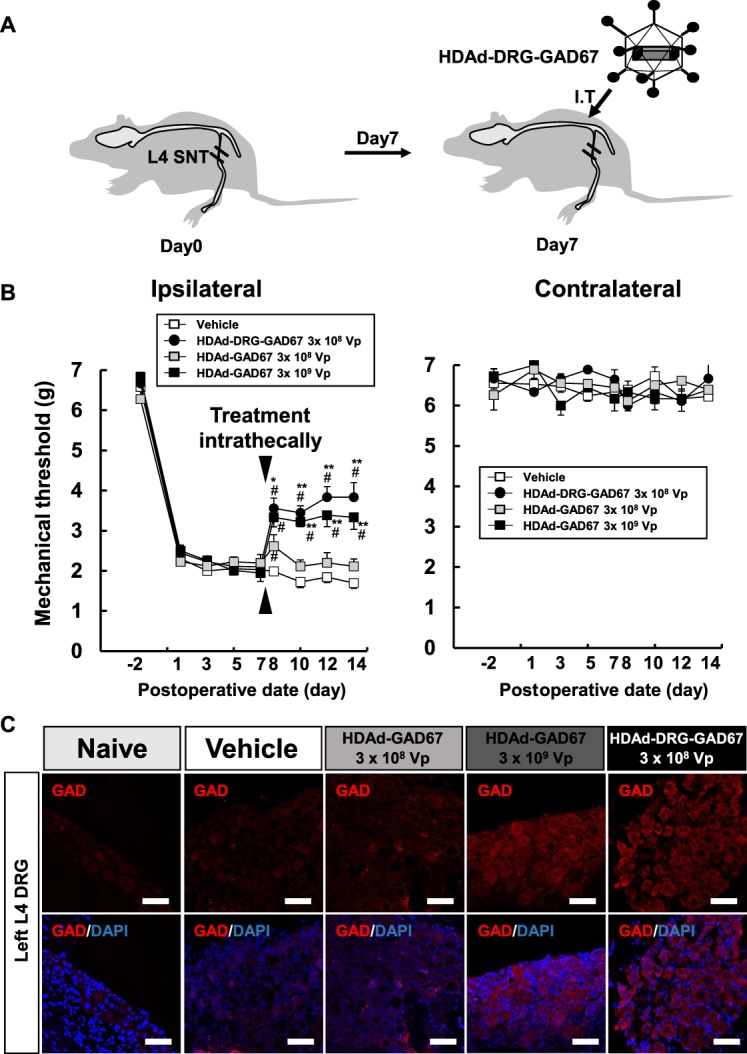
HDAd-DRG-GAD67 gene therapy ameliorates neuropathic pain induced by SNT. (A) Schematic presentation of treatment for SNT-induced neuropathic pain. Spinal nerve transection was performed on day 0 and treatment vectors were administrated intrathecally on day 7. Regular icosahedron indicates HDAd-DRG-GAD67. I.T., intrathecal injection. (B) The mechanical threshold on the bilateral hind paw was measured on days −2, 1, 3, 5, and 7 after SNT as pretreatment condition and days 8, 10, 12, and 14 after SNT as posttreatment condition (n = 5 per vehicle or HDAd-GAD67 3 × 108 Vp group, n = 6 per HDAd-DRG-GAD67 3 × 108 Vp or HDAd-GAD67 3 × 109 Vp group). (C) Immunohistochemistry of GAD in the injured L4 DRG on day 14 after SNT (day 7 after intrathecal treatment) with treatment. Upper panels show immunostainings of anti-GAD (red), and lower panels show merged images with DAPI (blue) staining. #P < 0.01 compared with the vehicle group, **P < 0.01, *P < 0.05 compared with the HDAd-GAD67 3 × 108 Vp group. Bars indicate mean ± SE. Scale bar = 50 μm. DRG, dorsal root ganglion; GAD, glutamic acid decarboxylase; HDAd, helper-dependent adenovirus; SNT, spinal nerve transection.
