Figure 1. Optogenetic manipulation of DA receptors D1 and D2 in the Sp5C differentially regulates trigeminal neuropathic pain.
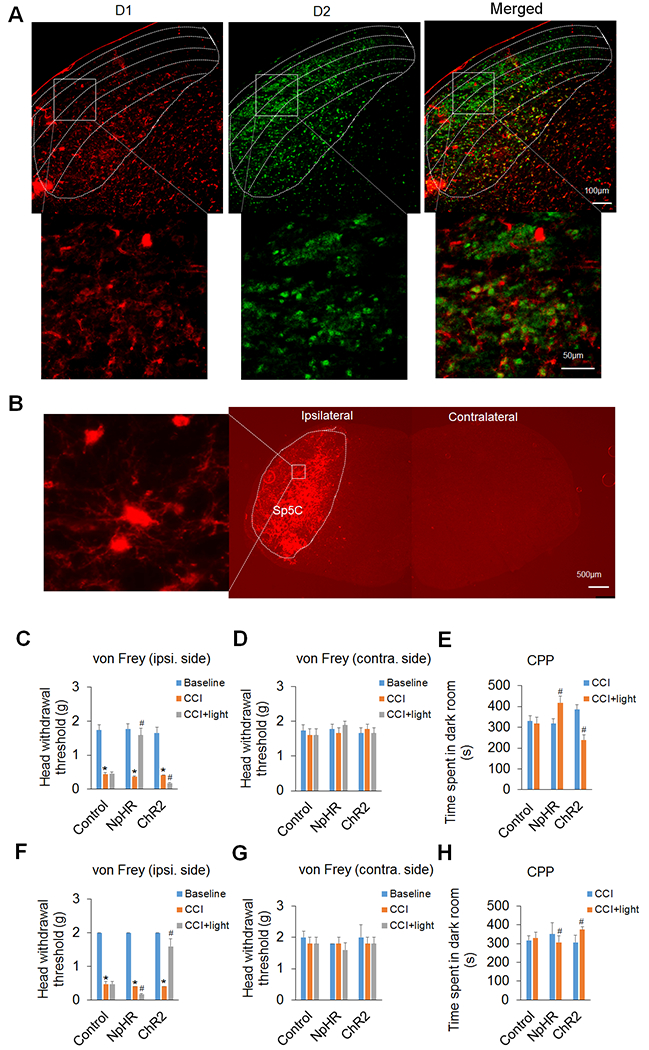
(A) Immunohistochemical staining showed that DA receptors D1 and D2 were expressed mostly in different neurons of the Sp5C. The upper panel displays the expression of D1 and/or D2 in the Sp5C at low magnification. Note that D1 was predominantly expressed in the deep laminae of the Sp5C and D2 was mostly expressed in the superficial laminae of the Sp5C. The lower panel displays the expression of D1 and/or D2 in the respective boxed areas of the upper panel at high magnification. (B) AAV5-mediated ChR2 expression in the Sp5C on day 14 after virus infusion into unilateral Sp5C represented that Cre-inducible viruses strongly expressed light-sensitive proteins on D1/2-expressing neurons in the ipsilateral Sp5C of D1-Cre or D2-Cre transgenic mice. The left image represents the boxed area in the ipsilateral Sp5C at high magnification. Note that there was no ChR2 expression in the contralateral Sp5C. (C–E) In D1-Cre mice, optogenetic inhibition of the Sp5C D1-expressing neurons significantly attenuated the CCI-ION-induced neuropathic pain on day 14 post-surgery and optogenetic excitation of the Sp5C D1-expressing neurons significantly increased such pain in the CCI-ION model. The von Frey filament test showed that inhibition of the Sp5C D1-expressing neurons with light stimulation of inhibitory opsin NpHR robustly increased head withdrawal threshold and excitation of the Sp5C D1-expressing neurons with light stimulation of excitatory opsin ChR2 significantly decreased the head withdrawal threshold in the ipsilateral side of the CCI-ION (C), though the optogenetic manipulation had no effect on the pain thresholds in the contralateral side of the CCI-ION (D). The CPP test showed that time spent in the dark room, which was paired with light stimulation, was significantly increased in the mice with the expression of NpHR in the Sp5C, but the time was markedly decreased in the mice with the expression of ChR2 in the Sp5C (E). Optogenetic stimulation did not significantly alter these behaviors in the D1-Cre mice with the expression of control virus in the Sp5C. (F–H) In D2-Cre mice, optogenetic inhibition of the Sp5C D2-expressing neurons significantly enhanced the CCI-ION-induced neuropathic pain on day 14 post-surgery and optogenetic excitation of the Sp5C D2-expressing neurons dramatically diminished such pain in both von Frey (F) and CPP (H) tests. Likewise, optogenetic stimulation had no effect on the pain thresholds in the contralateral side of the CCI-ION (G) and did not significantly alter these behaviors with the expression of control virus in the Sp5C. n = 6–7 mice per group. *P < 0.05 vs. the corresponding Baseline values; #P < 0.05 vs. the corresponding CCI group.
