Figure 3.
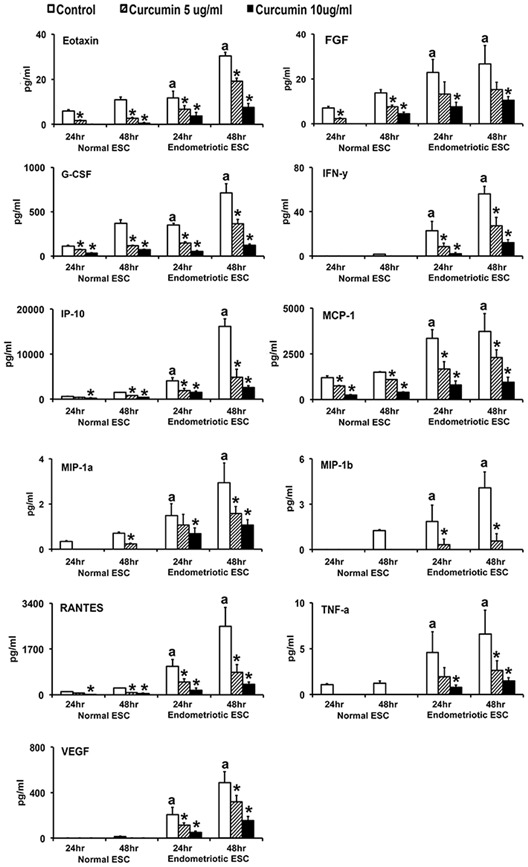
CUR attenuated proinflammatory chemokines and cytokines secreted by human NESCs and cells derived from EESCs. Cells were treated with or without CUR (5 µg/ml or 10 µg/ml) for 24 and 48 hr in DMEM/Ham’s F‐12 media with 5% exosome‐depleted fetal bovine serum. Concentrations of proinflammatory chemokines and cytokines were measured and analyzed in the supernatants using Bio‐Plex Pro Human Cytokine, Chemokine, and Growth Factor Magnetic Bead‐Based Assays, coupled with the Luminex 200 system (R&D System Inc., Minneapolis, MN). All bar graphs represent the mean ± SEM of results from three individual experiments (n = 3) from eutopic endometrial biopsies from three subjects with and three without evidence of endometriosis. The superscript “a” represents significant differences (p ≤ 0.05) in EESCs groups compared with respective NESCs groups at 24 and 48 hr. Star (*) represents significant differences (p ≤ 0.05) in EESCs groups treated with CUR compared with respective NESCs groups treated with CUR at 24 and 48 hr. CUR: curcumin; CCL11: chemokine eotaxin; DMEM: Dulbecco’s modified Eagle’s medium; EESC: eutopic endometrium of endometriosis subjects; FGF: fibroblast growth factors; G‐CSF: granulocyte‐colony stimulating factor; GM‐CSF: granulocyte‐macrophage colony stimulating factor; IFNγ: interferon γ; IP‐10/CXCL10: interferon γ‐induced protein 10; MCP‐1/CCL2: monocyte chemotactic protein‐1; MIP‐1a/CCL3: macrophage inflammatory proteins 1a; NESC: normal endometrial stromal cells; PDGF: platelet‐derived growth factor; TNF‐α: tumor necrosis factor‐α; VEGF: vascular permeability factor/vascular endothelial growth factor
