Abstract
The Epilepsy Bioinformatics Study for Anti-epileptogenic Therapy (EpiBioS4Rx) is a longitudinal prospective observational study funded by the National Institute of Health (NIH) to discover and validate observational biomarkers of epileptogenesis after traumatic brain injury (TBI). A multidisciplinary approach has been incorporated to investigate acute electrical, neuroanatomical, and blood biomarkers after TBI that may predict the development of post-traumatic epilepsy (PTE). We plan to enroll 300 moderate-severe TBI patients with a frontal and/or temporal lobe hemorrhagic contusion. Acute evaluation with blood, imaging and electroencephalographic monitoring will be performed and then patients will be tracked for 2 years to determine the incidence of PTE. Validation of selected biomarkers that are discovered in planned animal models will be a principal feature of this work. Specific hypotheses regarding the discovery of biomarkers have been set forth in this study. An international cohort of 13 centers spanning 2 continents will be developed to facilitate this study, and for future interventional studies.
SUMMARY
This is a comprehensive prospective study of biomarkers of post-traumatic epileptogenesis. The unique aspects of this study are 1) the early use of cEEG monitoring, 2) depth EEG monitoring, 3) study of both pediatric and adult subjects in a combined study, 4) a selective clinical phenotype which matches the experimental models.
INTRODUCTION
The development of post-traumatic epilepsy (PTE) has been the subject of previous observational studies1–4. However, a comprehensive study to validate translational biomarkers in a specific high risk traumatic brain injury (TBI) phenotype has heretofore not been performed. As addressed in our accompanying paper by Tubi et al, we have found a specific high risk TBI phenotype for PTE. We will plan to study unique bioelectrical biomarkers using electroencephalography (EEG), combined with imaging and blood biomarkers to evaluate the development of PTE.
Previous studies have identified an array of potential biomarkers for PTE. In adult male Sprague-Dawley rats, Bragin et al. demonstrated the detection of pathological high frequency oscillations (pHFOs) using scalp and depth continuous EEG (cEEG) within the first 7 days after fluid percussion injury (FPI)5. Rats that had early pHFOs and repetitive HFOs and spikes (rHFOSs) eventually experienced late seizures5. We will perform cEEG monitoring on Intensive Care Unit (ICU) patients in order to detect analogous early seizure activity. Correlating early findings with later PTE development will help improve ICU EEG monitoring and early intervention timing for epilepsy.
For structural markers, deformations in the ipsilateral hippocampus have been found within one week post injury in rats that develop late seizures, and human studies have shown that long-term ipsilateral hippocampal atrophy is associated with early seizure activity in the acute phase of TBI6,7. In addition, through T1 magnetic resonance imaging (MRI), perilesional cortex and thalamus changes in rats showed highest predictive value for subjects that demonstrated increased seizure susceptibility 12 months after injury8. We will correlate hippocampal and thalamic changes detected in MRI with epileptiform activity found in EEG and neurocognitive outcomes scored during follow up evaluations. We hope to understand the predictive ability of structural or functional MRI biomarkers for PTE in ICU patients.
Fluid biomarkers common in TBI have been identified, including tau and IL-1β9,10. However, their involvement in the epileptogenic process has not been fully characterized. As part of the EpiBioS4Rx project, two analogous animal projects will be performed alongside the clinical study to analyze blood plasma from rats at acute and chronic time points. The results from these projects will determine the biomarker panel for the human TBI cohort, and our study will attempt to validate particular metabolites that can be targeted with anti-epileptogenic drugs.
We hypothesize that early post-traumatic epileptic EEG activity within the first 7 days post TBI including seizures, periodic discharges, pHFOs, rHFOSs, and acute structural/functional abnormalities within hippocampal or thalami-cortical networks indicate the presence of an epileptogenic process in patients after moderate-severe TBI. In addition, we expect that PTE predicting acute plasma biomarkers informed by animal studies can be validated in ICU patients. And finally, the EpiBioS4Rx study will establish an online data repository for TBI and PTE, as well as an experienced ICU network for developing future anti-epileptogenic trials.
We have outlined a number of specific hypotheses including: 1) Early seizures within the first 7 days post TBI will occur in over 20% of this TBI phenotype, 2) Early seizures will predict the development of PTE, 3) Early pHFOs or rHFOSs will predict PTE, 4) Blood biomarkers of epileptogenesis that are discovered in animal models will manifest acutely in ICU patients when compared to up to 180 days after TBI, 5) Early structural or functional disconnections of hippocampal related or hippocampal-fugal fibers will indicate the development of epileptogenesis, and 6) Prospective implementation of high resolution advanced EEG methods among our TBI-ICU-EEG-study sites will enable the selection of an enriched population of patients at high risk based of developing PTE that can be targeted in a future interventional trial.
METHODS
Study Sites and Patient Population
The study sites include University of California, Los Angeles (UCLA – clinical coordinating center), University of California, Davis, Phoenix Childrens’ Hospital, Yale University, Harvard University/Massachusetts General Hospital, University of Pennsylvania, University of Cincinnati, University of Miami, University of Pittsburgh, Columbia University, Royal Melbourne Hospital, The Alfred, Children’s National Hospital. Figure 1 illustrates the entire course of the EpiBioS4Rx study, including both patient related study procedures and post-enrollment analysis. 300 total patients will be enrolled over 3 years, and they will be followed longitudinally for 2 years after injury. Patients admitted into the ICU after an acute moderate-severe TBI involving a frontal and/or temporal lobe hemorrhagic contusion will be screened. Patients are eligible for enrollment up to 72 hours post TBI. Criteria include ages 6 to 100 and Glasgow Coma Scale (GCS) 3 to 13. Patients are excluded for isolated diffuse axonal injury (DAI), isolated epidural or subdural hemorrhages, isolated anoxic brain injury, pregnancy, incarceration, and pre-existing neurodegenerative or epileptic disorders.
Figure 1:
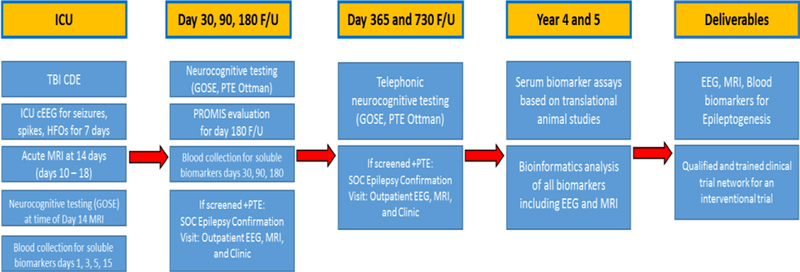
Outline of the entire course for the EpiBioS4Rx study
REDCap and LONI IDA Online Databases
ICU physiological data, demographic information, outcome measures, and prospective research data will be uploaded to the REDCap (Research Electronic Data Capture) data repository hosted by the University of Southern California Laboratory of Neuroimaging (USC LONI). REDCap is a secure, web-based application designed to support data capture for research studies. The online database contains 26 electronic case report forms (eCRFs) designed by the UCLA Brain Injury Research Center. Common data elements were originally culled from the TBI and Epilepsy case report forms provided by the National Institute of Health (NIH) Online Toolbox11, but expanded to collect EEG, MRI, and biosample information. Additional forms included the Glasgow Outcome Scale – Extended (GOS-E)12, ADAPT GOS-E Pediatrics Form13, Post-traumatic Epilepsy Ottman Questionnaire, and the Patient-Reported Outcomes Measurement Information System (PROMIS) profile assessments for adults, pediatrics, and parent proxies14. The GOS-E and PROMIS forms were incorporated by using existing electronic forms available through the REDCap Shared Library. The GOS-E pediatrics and PTE Ottman Questionnaire were newly created into REDCap and taken from the original paper forms. All cEEG and neuroimaging data across sites will be uploaded and managed by the USC LONI Image Data Archive (IDA).
Continuous Scalp and Depth EEG Monitoring to Detect Early Seizures
Enrolled patients will receive 24 hour cEEG for 72 hours minimum during the first 7 days after TBI. Scalp cEEG monitoring will be performed at the patient bedside using a 16–21 channel bipolar and referential composite montage implemented at each study center based on their established ICU EEG protocols. Mandatory parameters include low frequency filter (LFF) at 0.1 Hz, high frequency filter (HFF) at 50 Hz, Notch Filter on, and a 200 Hz minimum sampling rate. Each site will use their own standard of care electrodes, including disk or needle scalp electrodes. Study teams will input EEG settings and seizure event information into the REDCap database. A subset of 100 patients will receive additional depth EEG monitoring during the first 7 days after TBI for higher resolution and pHFOs/rHFOSs detection. Sites will use a mini depth 6 channel electrode along with a referential montage at 0.1 Hz LFF, 50 Hz HFF, Notch Filter on, and 2000 Hz sampling rate. Rhythm-link corkscrew electrodes will be used at all sites to reduce background noise caused by possible multimodality monitoring. 24 hour cEEG files will be de-identified and uploaded to the LONI IDA. Study sites will perform a pre-study sample EEG that will be reviewed by the central analysis team to validate EEG quality.
The central analysis team will review cEEG data using PERSYST version 13 and Matlab 8.1 to analyze spikes, pHFOs, and rHFOSs. A structured protocol will be performed to determine interictal epileptiform spike and seizure onset, location of epileptiform onset, spike morphology, spike repetition rate, clustering features, field size, and spread patterns. EEG data will be correlated with MRI imaging data and metabolite plasma data over the first 7 days of injury.
Multimodal MRI Analysis for Structural Biomarkers
The initial three standard of care clinical CT scans over the first 24 hours after trauma will be de-identified and uploaded to the LONI IDA to confirm initial injury characteristics. A high-resolution MRI, acquired on a 3T scanner, will be performed on Day 14 (± 4 days) post-injury. MRI sequences acquired include: 3D T1, 2D resting state bold oxygen level dependent imaging (rs- BOLD), 2D diffusion tensor imaging (DTI), 3D Gradient Echo / Susceptibility Weighted Imaging (GRE/SWI), 3D T2, 3D T2-weighted fluid attenuated inversion recovery (FLAIR). MRI acquisition parameters will be optimized across sites and scanner types to reduce inter-scanner variability. Additionally, study sites will perform a preliminary phantom scan, which will be reviewed by the central analysis team to ensure mandatory resolution requirements are met.
MRI injury location and total hemorrhagic lesion load will be correlated with PTE occurrence. T1-weighted MRI scans will be used for 1) a regional volumetric analysis, using FSL utilities, and a 2) subcortical morphometric shape analysis, using a validated open-source pipeline robust to brain pathology. Disruption of thalamo-cortical and hippocampal structural connectivity at the global level will be assessed for each subject using diffusion tensor connectomics and correlated with PTE incidence. After conventional preprocessing (e.g., slice timing correction, motion correction, smoothing, band-pass filtering) of rs-fMRI, functional connectivity will be assessed through seed-based analysis (making use of patient-specific segmentations of hippocampus and thalamus as seeds), and graph theoretic analysis. Each of these analyses incorporate additional processing steps for modeling of white matter, cerebrospinal fluid, global mean DTI signal, extended motion parameters, and single-spike modeling. In an exploratory analysis, we will perform a broader screen of injury types (DAI vs. subdural hemorrhage).
Biosample Collection and Analysis
Study sites will collect blood samples through central lines or venous punctures on post-injury day 1, 3, 5, 15, 30 ± 10, 90 ± 10 , and 180 ± 10. Samples will be centrifuged at 1500 × g in 4 °C for 15 minutes, and plasma will be aliquoted. Sites will ship plasma on dry ice to the BioSEND biostorage facility at Indiana University for storage and maintenance. Biosample collection, processing, and shipping information will be inputted into the REDCap database. BioSEND will distribute samples to laboratories in the Uniformed Services University for metabolomics analysis. The specific biomarker panel tested for the human TBI cohort will be determined by the results of the two animal projects that coincide with this study. Our anticipated panel includes neuron specific enolase (NSE), creatine kinase BB (CK-BB), S100 calcium binding protein B (S100B), glial fibrillary acidic protein (GFAP), myelin basic protein (MBP), phosphorylated tau (p-tau), neurofilament-H (NF-H), interleukin-1 beta (IL-1β), interleukin-6 (IL-6), interleukin-8 (IL-8), tumor necrosis factor-alpha (TNF-), interferon gamma (INF-y), Ceruloplasmin, hypoxia-inducible factor 1 alpha (HIF-1a). cEEG and/or depth EEG data will be correlated to the biomarker results in order to characterize the relationship between EEG epileptiform activity and appearance and time course of selected biomarkers.
Neurocognitive Outcome Measures and PTE Confirmation
Patients will undergo scheduled neurocognitive outcome evaluations over 2 years post-TBI. The adult or pediatric Glasgow Outcome Scale - Extended (GOS-E)12,13 and the Ottman Post-Traumatic Epilepsy Questionnaire will be performed on post-injury day 30, 90, 180, 365, and 730. The adult or pediatric GOS-E will also be performed on post-injury day 14 alongside the acute MRI. The PROMIS Profile Adults, Pediatrics, or Parent Proxy Forms14 will be completed on injury day 180 only. If a patient reports recurrent seizure activity during the follow-up periods, the patient will undergo a 30 minute outpatient sleep/wake EEG and an additional Epilepsy Confirmation Clinic Interview by a site epileptologist to confirm PTE diagnosis. All longitudinal follow up evaluations will be uploaded to the REDCap database.
Power Analysis
In this study, we propose to enroll 300 subjects. For the concept of whether early seizures or epileptiform activity predict PTE, the number to determine if early seizures (recorded on depth or surface) predict late PTE, based on a 50% incidence of early seizures and 50% incidence of PTE (in this highly selected group) was n=144. Assuming a 30% attrition rate, initial n=187. For HFO detection, we have only animal data to consider for power estimates, with 60% of animals having HFOs early after TBI. We anticipate incomplete overlap between early seizures and HFOs, hence an additional 100 subjects are being added empirically for depth EEG, pHFO, and rHFOSs studies. For the imaging aims, we powered the MRI studies based on our existing work regarding temporal lobe injury being prevalent in the PTE group (74% in PTE vs. 35% in non-PTE; effect size h=0.805, total n=120, or initial n before attrition = 172). For the blood biomarker study aims, we used results from animal studies of the effect of PTE on tau (Tai et al, 2016) and the effects of sodium selenate to mitigate seizures and tau expression15. We also used existing human studies of serum tau levels after TBI to model the expected increase in serum tau that occurs due to trauma complicated by seizures. We powered the study in order to demonstrate between group differences in those patients with severe TBI plus seizures vs. those with severe TBI alone during the initial week after TBI. Based on published values for serum tau (ranging from 10 pg/ml (SD 15) in mild TBI to 436 pg/ml (SD 472), the number of subjects needed was n=108. With 30% attrition, n= 144.
Study Administration
The study features near-real time enrollment supervision by the clinical coordinating center, UCLA, to ensure compliance with phenotype requirements for enrollment. Weekly study teleconferences are conducted to review enrollments, protocol revisions, and data flow. Blood Biosamples are collected, stored and analyzed for quality control by BioSEND® at the University of Indiana, and analyzed by the laboratory of Dr. Denes Agoston at Uniformed Services University. Periodic in-person meetings are held to discuss scientific issues. A steering committee of site principal investigators and study co-investigators review scientific and business issues. Review of study progress with the funding agency program officers occurs bi-weekly. The Data and Safety Monitoring Board (DSMB), led by Dr. Bleck, reviews all serious adverse events (SAEs) on a quarterly basis.
Study Limitations
Limitations to the EpiBioS4Rx study include variation in length of time between patient screening and enrollment. Patients who are not enrolled on the day of injury may miss non-standard of care procedures on day 1, including the blood biosample. However, all data collected per standard of care should be available even if the patient is not consented immediately. For EEG, standard of care cEEG procedures vary across enrolling sites. Either scalp and/or depth cEEG may be applied at varying times during the first 7 days post TBI, depending on clinical indication. Other limitations include self-reporting of seizures and neurocognitive outcomes during Day 14, 30, 90, 180, 365, and 730 follow up visits. Case report forms were developed to standardize patient responses, but responses are still based on patient’s or caregiver’s recollection of events. In addition, patients may primarily report obvious seizure occurrences, and not seizure activity that they may be unaware of. The PTE Ottman Questionnaire, Epilepsy Confirmation Clinic Interview, and Outpatient EEG/MRI procedures are in place to help standardize the PTE diagnosing process across patients and sites. They will address the nature of symptoms experienced, the number of occurrences, and any treatment given prophylactically or post seizures.
Acknowledgments
Funding: NINDS Center without Walls, U54 NS100064 (EpiBioS4Rx)
ABBREVIATIONS
- ADAPT
Approaches and Decisions in Acute Pediatric TBI
- BioSEND
Biospecimen Exchange for Neurological Disorders
- cEEG
Continuous Electroencephalography
- CDE
Common Data Elements
- CK-BB
Creatine Kinase BB
- CT
Computed Tomography
- DAI
Diffuse Axonal Injury
- DSMB
Data and Safety Monitoring Board
- DTI
Diffusion Tensor Imaging
- eCRFs
Electronic Case Report Forms
- EpiBioS4Rx
Epilepsy Bioinformatics Study for Anti-epileptogenic Therapy
- FLAIR
Fluid Attenuated Inversion Recovery Imaging
- FPI
Fluid Percussion Injury
- GFAP
Glial Fibrillary Acidic Protein
- GCS
Glasgow Coma Scale
- GOS-E
Glasgow Outcome Scale – Extended
- GRE
Gradient Echo Imaging
- HFF
High Frequency Filter
- HIF-1α
Hypoxia-inducible Factor – 1 alpha
- Hz
Hertz
- ICU
Intensive Care Unit
- IL-1β
Interleukin – 1 Beta
- IL-6
Interleukin – 6
- IL-8
Interleukin – 8
- INF-γ
Interferon gamma
- LFF
Low Frequency Filter
- LONI IDA
Laboratory of Neuroimaging Imagine Data Archive
- MRI
Magnetic Resonance Imaging
- MBP
Myelin Basic Protein
- NF-H
Neurofilament - H
- NIH
National Institute of Health
- NSE
Neuron Specific Enolase
- pHFOs
Pathological High Frequency Oscillations
- PROMIS
Patient-Reported Outcomes Measurement Information System
- PTE
Post-traumatic Epilepsy
- p-tau
Phosphorylated tau
- REDCap
Research Electronic Data Capture
- rHFOSs
Repetitive High Frequency Oscillations and Spikes
- rs-BOLD
Resting State Bold Oxygen Level Dependent Imaging
- SAE
Serious Adverse Event
- SD
Standard Deviation
- SWI
Susceptibility Weighted Imaging
- S100B
S100 Calcium Binding Protein B
- T
Tesla
- TBI
Traumatic Brain Injury
- TNF-α
Tumor Necrosis Factor - alpha
- T1
Longitudinal Relaxation Time
- T2
Transverse Relaxation Time
- UCLA
University of California, Los Angeles
- USC LONI
University of Southern California Laboratory of Neuroimaging
- 2D
Two Dimensional
- 3D
Three Dimensional
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Englander J, Bushnik T, Duong TT, et al. Analyzing risk factors for late posttraumatic seizures: a prospective, multicenter investigation. Arch Phys Med Rehabil 2003;84:365–373. [DOI] [PubMed] [Google Scholar]
- 2.Asikainen I, Kaste M, Sarna S. Early and late posttraumatic seizures in traumatic brain injury rehabilitation patients: brain injury factors causing late seizures and influence of seizures on long-term outcome. Epilepsia 1999;40:584–589. [DOI] [PubMed] [Google Scholar]
- 3.Chen W, Li MD, Wang GF, Yang XF, Liu L, Meng FG. Risk of post-traumatic epilepsy after severe head injury in patients with at least one seizure. Neuropsychiatr Dis Treat 2017;13:2301–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Annegers JF, Hauser WA, Coan SP, Rocca WA. A population-based study of seizures after traumatic brain injuries. N Engl J Med 1998;338:20–24. [DOI] [PubMed] [Google Scholar]
- 5.Bragin Anatol et al. “Pathologic Electrographic Changes after experimental Traumatic Brain Injury.” Epilepsia 57.5 (2016): 735–745. PMC. Web. 31 Jan. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shultz, Sandy R et al. “Can Structural or Functional Changes Following Traumatic Brain Injury in the Rat Predict the Epileptic Outcome?” Epilepsia 54.7 (2013): 1240–1250. PMC. Web 31 Jan 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vespa PM et al. “Nonconclusive Seizures after Traumatic Brain Injury Are Associated with Hippocampal Atrophy.” Neurology 75.9 (2010): 792–798. PMC. Web 31 Jan. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Immonen Riikka et al. “MRI Biomarkers for Post-Traumatic Epileptogenesis.” Journal of Neurotrauma 30.14 (2013): 130501309. PMC. Web 31 Jan. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liliang PC, Liang CL, Weng HC, Lu K, Wang KW, Chen HJ, Chuang JH. Tau proteins in serum predict outcome after severe traumatic brain injury. J Surg Res 2010. May 15;160(2):302–7 [DOI] [PubMed] [Google Scholar]
- 10.Diamond, Matthew L et al. “IL-1β Associations with Post-Traumatic Epilepsy Development: A Genetics and Biomarker Cohort Study.” Epilepsia 55.7 (2014): 1109–1119. PMC. Web. 31 Jan. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gershon Richard C et al. “NIH Toolbox for Assessment of Neurological and Behavioral Function.” Neurology 80.11 Suppl 3 (2013): S2–S6. PMC. Web. 31 Jan. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson Lindsay JT, Pettigrew LEL, Teasdale GM Journal of Neurotrauma. August 1998, 15(8): 573–585. 10.1089/neu.1998.15.573 [DOI] [PubMed] [Google Scholar]
- 13.Beers Sue R et al. “Validity of a Pediatric Version of the Glasgow Outcome Scale–Extended.” Journal of Neurotrauma 29.6 (2012): 1126–1139. PMC. Web. 28 Feb. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, Amtmann D, Bode R, Buysse DJ, Choi SW, Cook KF, DeVellis R, DeWalt D, Fries JF, Gershon R, Hahn E, Pilkonis P, Revicki D, Rose M, Weinfurt K, & Hays RD on behalf of the PROMIS Cooperative Group. (2010). Initial item banks and first wave testing of the Patient–Reported Outcomes Measurement Information System (PROMIS) network: 2005–2008. Journal of Clinical Epidemiology, 63(11), 1179–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tai XY, Koepp MJ, Duncan JS, Fox NC Thompson PJ, Baxendale SA, Liu JY, Reeves CA, Michalak Z, & Thom M (2016). Hyperphosphorylated tau in patients with refractory epilepsy correlates with cognitive decline: a study of temporal lobe resections. Brain : a journal of neurology, 139 Pt 9, 2441–55. [DOI] [PMC free article] [PubMed] [Google Scholar]


