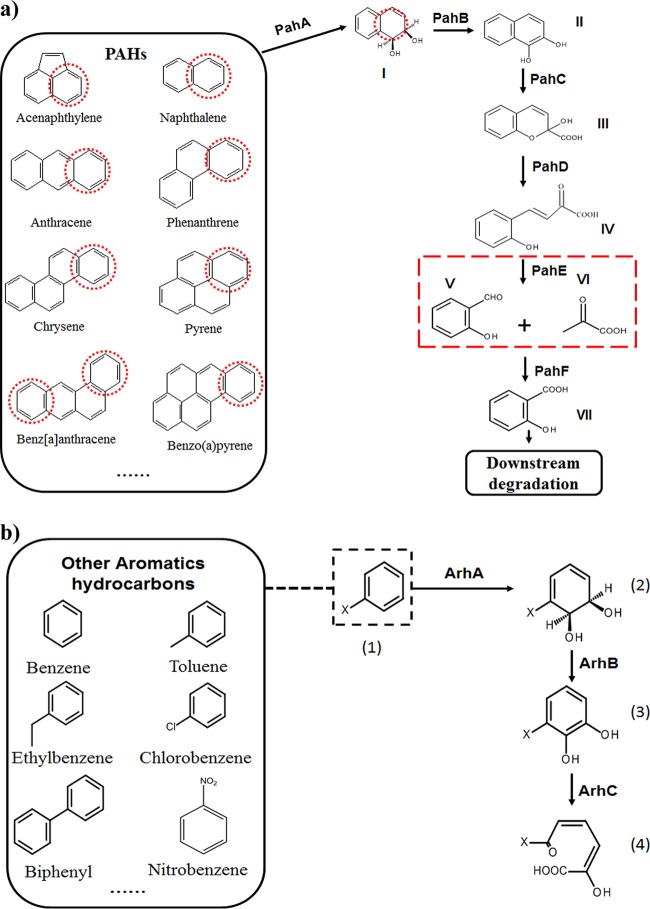
FIG 1.
The upper consensus metabolic pathway of PAHs, with naphthalene as an example (a), and other aromatic hydrocarbons, with x-benzene as an example (b). Chemicals are I, cis-1,2-dihydroxy-1,2-dihydronaphthalene; II, 1,2-dihydroxynaphthalene; III, 2-hydroxy-4-(2′-oxo-3,5-cyclohexadienyl)-buta-2,4-dienoate; IV, trans-o-hydroxybenzylidenepyruvate; V, salicylaldehyde; VI, pyruvate; VII, salicylate; (1), x-benzene; (2), cis-5,6-dihydroxy-5,6-dihydro-1-x-benzene; (3), 5,6-dihydroxy-1-x-benzene; (4), 2-hydroxyl-6-oxo-6-x-2,4-diadienic acid. Enzymes are PahA, naphthalene dioxygenase; PahB, cis-1,2-dihydroxy-1,2-dihydronaphthalene dehydrogenase; PahC, 1,2-dihydroxynaphthalene dioxygenase; PahD, 2-hydroxychromene-2-carboxylate isomerase; PahE, trans-o-hydroxy- benzylidene pyruvate hydratase-aldolase; PahF, salicylaldehyde dehydrogenase; ArhA, aromatic hydrocarbon ring-hydroxylating dioxygenase; ArhB, aromatic hydrocarbon dihydrodiol dehydrogenase; ArhC, aromatic hydrocarbon extradiol dioxygenase. Red dotted circle, activated aromatic ring; red dotted box, the reaction catalyzed by PahE; black dotted box, x-benzene as an example.