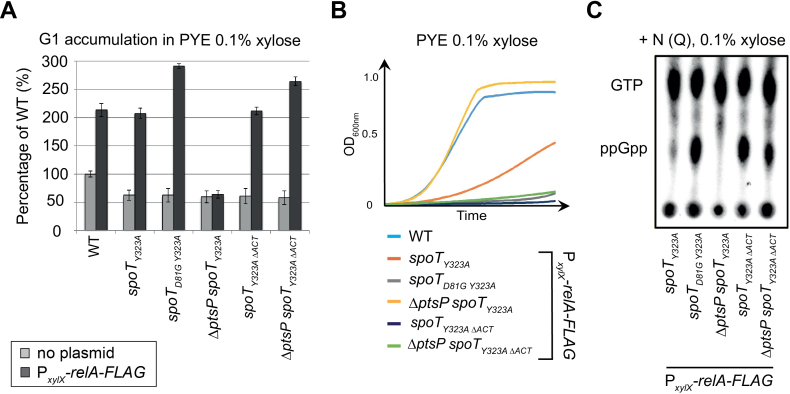
Figure 3.
The ACT domain is required in vivo to support the hydrolase activity of SpoT. (A and B) Absence of the ACT domain of SpoT promotes G1 accumulation and decreases growth rate upon artificial exogenous production of (p)ppGpp. (A) Flow cytometry analysis to determine DNA content in asynchronous population of WT (RH50), spoTY323A(RH1844), spoTD81GY323A(RH2193), ΔptsP spoTY323A (RH2196), spoT Y323AΔACT(RH1586) and ΔptsP spoTY323AΔACT(RH2492) strains with (black bars) or without (grey bars) PxylX::relA-FLAG grown for 6 h in PYE medium supplemented with 0.1% of xylose. The flow cytometry data were normalized to the WT without PxylX::relA-FLAG (100%). (B) The growth of strains used in (A) was measured for 24 h in PYE medium supplemented with 0.1% of xylose. Error bars = SD, n = 3. (C) The regulatory ACT domain of SpoT is required in vivo to degrade (p)ppGpp in nitrogen-replete condition (+N). The intracellular levels of (p)ppGpp were evaluated by TLC after nucleotides extraction from spoTY323A(RH1844), spoTD81GY323A(RH2193), ΔptsP spoTY323A (RH2196), spoTY323AΔACT(RH1586) and ΔptsP spoTY323AΔACT(RH2492) strains harbouring PxylX::relA-FLAG and grown for 2 h in glutamine (Q) containing media (+N) supplemented with 0.1% xylose.