Figure 6.
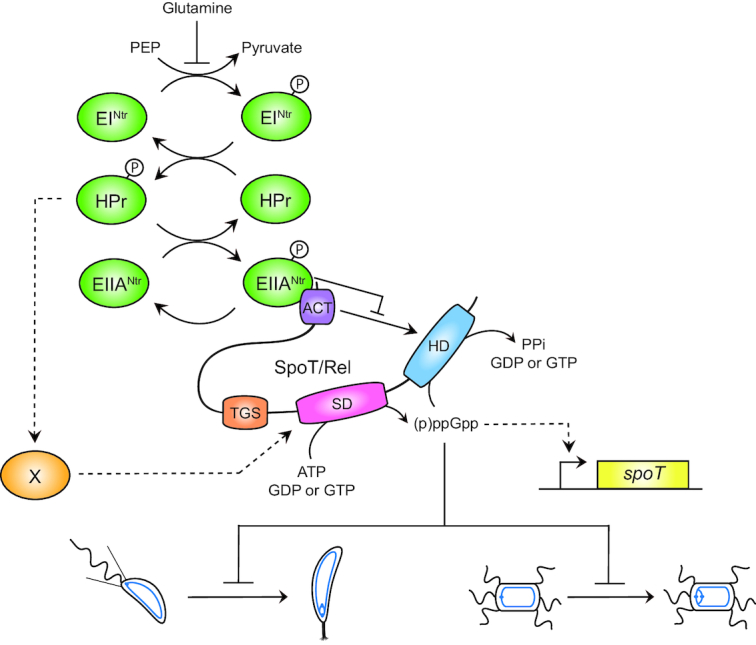
EIIANtr∼P binds the ACT domain of SpoT and inhibits its hydrolase activity. Upon nitrogen starvation (i.e. glutamine deprivation), EINtr phosphorylates its downstream components, HPr and EIIANtr. As a consequence, EIIANtr∼P interacts with ACT to likely inhibit its stimulating effect on the hydrolase activity of SpoT. This inhibition avoids to degrade (p)ppGpp produced by the SD, this latter being strongly stimulated by HPr∼P by an unknown mechanism. In addition, increasing the (p)ppGpp level promotes the accumulation of the SpoT protein by a positive feedback loop mechanism on transcription of the spoT gene. Ultimately, the burst of (p)ppGpp delays the G1-to-S transition of C. crescentus (left) and S. meliloti (right) cells.
