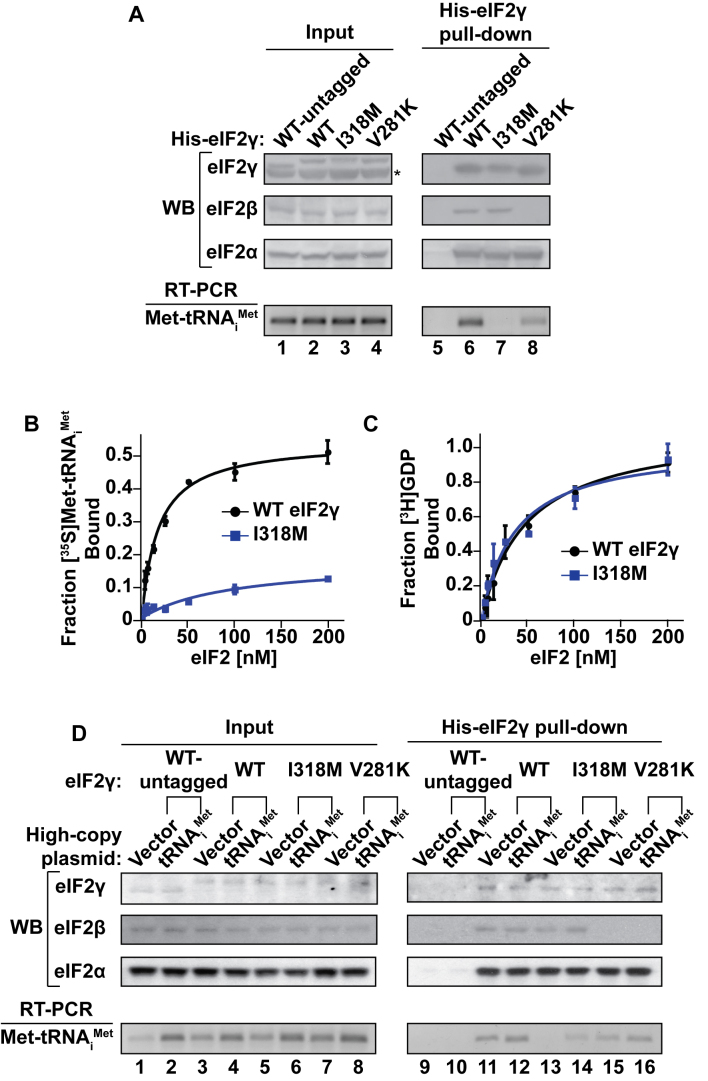
Figure 5.
The eIF2γ-I318M mutation reduces eIF2–GTP–Met-tRNAiMet TC formation. (A) WCEs from yeast strains expressing the indicated WT or mutant forms of untagged or His-tagged eIF2γ were either immediately processed (Input; lanes 1–4) or incubated with Ni-NTA resin (His-eIF2γ pull-down; lanes 5–8). Input and pull-down protein samples were subjected to SDS-PAGE followed by immunoblotting (western blot, WB) with rabbit polyclonal antisera against yeast eIF2γ, eIF2β and eIF2α. Input and pull-down RNA samples were subjected to RT-PCR using oligonucleotide primers specific to yeast tRNAiMet, and reaction products were resolved by gel electrophoresis. The asterisk (*) indicates a non-specific immunoblot band migrating faster than eIF2γ. (B and C) [35S]Met-tRNAiMet plus non-hydrolyzable GDPNP (B) or [3H]GDP (C) were incubated with increasing concentrations of purified eIF2 complexes containing WT eIF2γ (black circles) or eIF2γ-I318M (blue squares), followed by vacuum filtration. Each data point represents the mean and error bars indicate the standard deviation for the fraction of [35S]Met-tRNAiMet or [3H]GDP bound to eIF2 in nitrocellulose filter bindings assays from three independent experiments. (D) WCEs from yeast strains expressing the indicated WT or mutant forms of untagged or His-tagged eIF2γ with or without overexpression of tRNAiMet were either immediately processed (Input; lanes 1–8) or incubated with Ni-NTA resin (His-eIF2γ pull-down; lanes 9–16). Input and pull-down protein samples were subjected to SDS-PAGE followed by immunoblotting (western blot, WB) with rabbit polyclonal antisera against yeast eIF2γ, eIF2β or eIF2α. Input and pull-down RNA samples were subjected to RT-PCR using oligonucleotide primers specific to yeast tRNAiMet, and reaction products were resolved by gel electrophoresis.