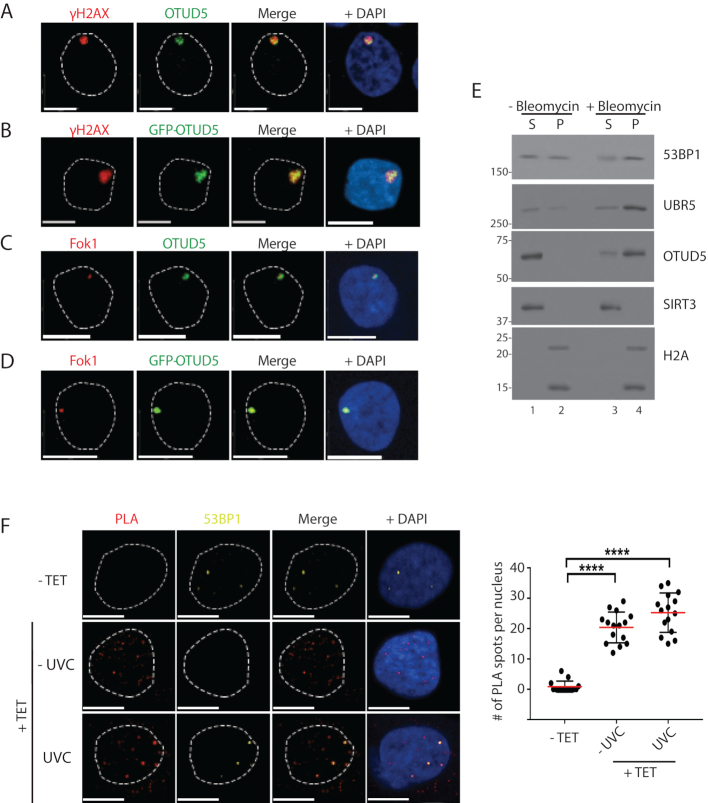
Figure 2.
OTUD5 localizes to UV and DSB-induced chromatin lesions. (A) HeLa cells were irradiated with UVC (100J/m2) through 3 µm micropore filters, and 1 hour later cells were fixed and co-stained with anti-γH2AX and OTUD5 antibodies. (B) HeLa cells were transfected with GFP-OTUD5 plasmid, irradiated with UVC through micropore filter as in A, and the fixed cells were stained for γH2AX. (C) mCherry-LacI-Fok1 fusion proteins (expressed in PTuner 263 U2OS cells) were induced by Shield-1 and 4-OHT (see ‘Materials and Methods’ section), and the fixed cells were stained with endogenous OTUD5 antibody. (D) The PTuner U2OS reporter cells were transfected with GFP-OTUD5. (E) 293T cells treated with or without Bleomycin (5 µM, 16 hours) were subject to fractionation assay (S = NaCl 100 mM eluate, P = the rest of pellet containing chromatin fraction) and the eluates were analyzed by western blots. (F) HeLa cells stably expressing Dox-inducible FLAG-OTUD5 were treated with Tetracyclin (10 µg/ml), followed by UV (30 J/m2) irradiation through micropore (3 µm) filter. PLA was performed with anti-FLAG and anti-UBR5 antibodies (see ‘Materials and Methods’ section). The slides were also co-stained with anti-53BP1 antibody to mark the DSB lesions. On the right is quantification for relative number of interactions per nucleus (N = 17). Scale bars indicate 10 μm. (**** indicates P-value <0.0005). Experiments were done in triplicates.