Figure 2. Dkk2 Is Specifically Upregulated In Plantar Dermis of Mouse but Not Rabbit Embryos.
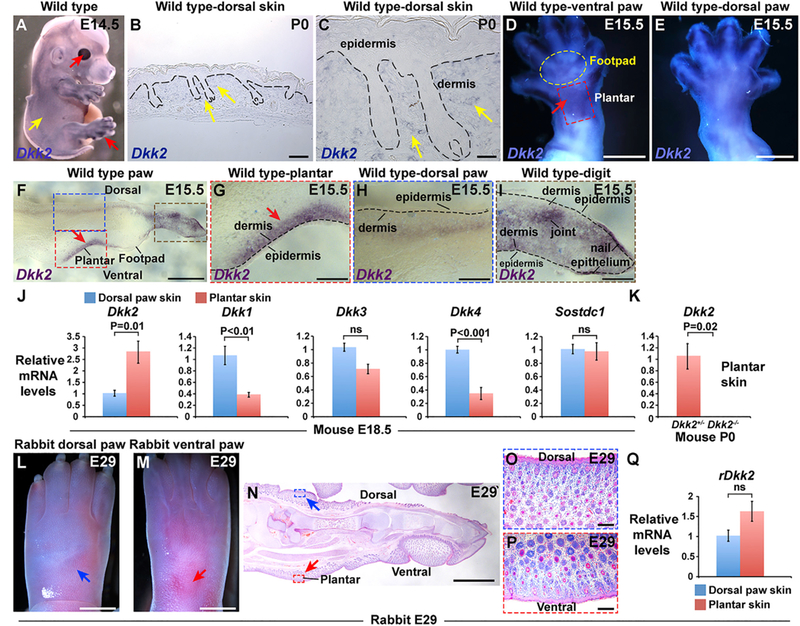
(A) Whole mount in situ hybridization for Dkk2 in wild-type mouse embryo at E14.5 (purple signal) reveals high expression levels in cornea and digits (red arrows) and low levels of expression in trunk and head skin between, but not within, hair follicle placodes (yellow arrow) (n = 6 biological replicates). (B) In situ hybridization for Dkk2 in sectioned wild-type mouse P0 dorsal skin (dark blue signal). (C) Higher magnification view of the area indicated by arrows in (B). Note low level expression in inter-follicular dermis (yellow arrows) (n = 3 biological replicates in B and C). (D and E) Ventral (D) and dorsal (E) views of an E15.5 mouse hind limb subjected to whole mount in situ hybridization for Dkk2 (purple-blue signal). Note high expression in the plantar region of the mouse ventral hind limb at E15.5 (arrow in D) (n = 4). (F) Cryosectioned mouse E15.5 hind limb following whole mount in situ hybridization for Dkk2 (purple signal) (n = 5 biological replicates). (G-I) higher magnification views of areas of (F) outlined with a dashed red box (G), a dashed blue box (H), and a dashed brown box (I). Note specific expression of Dkk2 in plantar dermis (arrows in F and G) compared with adjacent footpad skin and dorsal limb dermis at E15.5; low to undetectable levels of Dkk2 in dorsal limb dermis (H); and stronger signal in tissue located at a deeper level in the limb (H). In the limb digit, Dkk2 expression localizes to ventral but not dorsal dermis; relatively high levels of Dkk2 expression are also observed in nail epithelium and in the developing joint region (I). Dashed black lines in (B), (C), and (G)-(I) represent the dermal-epidermal border. (J) Relative levels of Dkk2, Dkk1, Dkk3, Dkk4, and Sostdc1 mRNA in full thickness dorsal paw skin (blue boxes) and plantar skin (pink boxes) from E18.5 mouse embryos, analyzed by qPCR (n = 5 plantar skin samples and n = 5 dorsal paw skin samples in each experiment). Note that only Dkk2 is specifically enriched in plantar skin. (K) Relative levels of Dkk2 mRNA in full thickness mouse Dkk2+/− control (pink box) and Dkk2−/−mutant (black box) plantar skin at P0, analyzed by qPCR (n = 3 littermate controls and n = 3 mutants). Dkk2 transcript levels are undetectable in Dkk2−/− skin. (L and M) Light microscopy of dorsal (L) and ventral (M) left hind paw of a rabbit embryo at E29. Developing hair follicles are present in the ventral plantar region (red arrow, M), as well as in dorsal skin (blue arrow, L). (N) Histology of embryonic rabbit left hind paw at E29 showing the presence of developing hair follicles in ventral plantar skin (red arrow) as well as dorsal paw skin (blue arrow). (O and P) Higher magnification views of the areas indicated in (N) by blue (O) and red (P) dashed boxes, respectively (5 biological replicates for L-P). (Q) Relative levels of rDkk2 mRNA in full thickness dorsal paw skin (blue box) and plantar skin (pink box)from E29 rabbit embryos, analyzed by qPCR (n = 5 plantar skin samples and n = 5 dorsal paw skin samples). Levels of rDkk2 mRNA are not significantly different between embryonic rabbit dorsal and plantar paw skin. All qPCR assays were performed in triplicate for each sample and data were normalized to Gapdh; mean values ± SD are shown. Statistical significance was calculated using Student’s t test. Scale bars, 100 μm (B); 30 μm (C); 750 μm (D and E); 470 μm (F); 150 μm (G-I, O, and P); 3 mm (L and M); 2 mm (N). See also Figure S2.
