Abstract
Background:
It is unclear whether exposure to polycyclic aromatic hydrocarbons (PAH) affects lung function in children with asthma. Whether vitamin D insufficiency enhances any detrimental effects of PAH on lung function in asthmatic children is also unknown.
Methods:
Cross-sectional study of 2,459 children (6-17 years) who participated in the 2007-2012 National Health and Nutrition Examination Survey. Multivariable linear regression was used to analyze the relation between molar mass of urinary PAH metabolites [sum of all PAH (ΣmolPAH), sum of PAH with 2 benzene rings (Σmol2-PAH), or sum of PAH with 3 or 4 benzene rings (Σmol3,4-PAH)] and lung function or exhaled fraction of nitric oxide (FeNO) in children with and without asthma. In this multivariable analysis, we tested whether vitamin D insufficiency (a serum 25(OH)D level <30 ng/ml) interacts with PAH exposure on lung function in children with asthma.
Results:
Among children with asthma, higher ΣmolPAH and Σmol2-PAH were significantly associated with decreased %predicted FEV1 (by 3.5%-3.8%). Urinary PAH were not associated with lung function in children without asthma or with FeNO. Given a significant interaction between vitamin D insufficiency and PAH metabolites on lung function in asthmatic children, we stratified the analysis by vitamin D status. In this analysis, urinary PAH metabolites were significantly associated with 2.0%-5.6% reduced %predicted FEV1 and %predicted FEV1/FVC in children with asthma and vitamin D insufficiency, but not in those with asthma and vitamin D sufficiency.
Conclusions:
Vitamin D insufficiency and PAH exposure may have synergistic detrimental effects on lung function in asthmatic children.
Keywords: polycyclic aromatic hydrocarbons, asthma, lung function, children, NHANES
INTRODUCTION
Asthma is one of the most common chronic diseases of childhood1. The prevalence of childhood asthma has substantially increased in industrialized countries over the last few decades, likely due to environmental changes2. Considerable evidence links exposure to air pollutants (such as particulate matter, mixed traffic-related air pollution, and polycyclic aromatic hydrocarbons (PAH)) to asthma in children3,4.
PAH, a group of hydrocarbons with two or more fused aromatic rings, are byproducts of incomplete combustion of tobacco, wood, coal, fossil fuels, and other organic substances. PAH can be readily absorbed through the skin, lungs, and gastrointestinal tract5. PAH metabolites in urine have been used as biomarkers of PAH exposure6. Naphthalene, a PAH compound consisting of 2 aromatic rings, is the most volatile PAHs found in the air and can be a respiratory tract toxicant7. PAH compounds consisting of 3-4 aromatic rings can exist in both gaseous and particulate phase5.
PAH exposure has been associated with asthma or asthma symptoms in children8-10. Although several studies have also linked PAH exposure to reduced lung function measures [(forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC)] among children and adults in the general population11-13, little is known about PAH exposure and lung function or eosinophilic airway inflammation in children with asthma, who may be particularly susceptible to the detrimental effects of air pollutants. In the only study examining PAH exposure and lung function among children with and without asthma, exposure to the sum of PAH with 4, 5, or 6 rings for 3 months to 1 year was significantly associated with reduced post-bronchodilator FEV1 among 162 children without asthma, but not among 135 children with asthma14. Underlying mechanisms for the PAH-asthma association include increasing oxidative stress, stimulating inflammatory response, enhancing sensitization to aeroallergens, and epigenetic remodeling4,15.
In addition to asthma, vitamin D insufficiency may modify the detrimental effects of PAH exposure on lung function, a marker of asthma severity. Indeed, vitamin D insufficiency has been previously associated with reduced lung function in the U.S. population16, and we previously reported that proximity to a major highway (a broad marker of traffic-related air pollution) is more significantly and strongly associated with severe asthma exacerbations in children with asthma and vitamin D insufficiency (a circulating 25(OH)D level <30 ng/ml) than in those with asthma and sufficient vitamin D levels17.
We hypothesized that PAH exposure would have a stronger detrimental effect on lung function and eosinophilic airway inflammation [measured by fractional exhaled nitric oxide (FeNO)] in children with asthma than in those without asthma. We further hypothesized that any detrimental effects of PAH exposure on the lung function of children with asthma would be stronger in those with vitamin D insufficiency than in those with sufficient vitamin D levels. To test these hypothesis, we first examined the relation between urinary PAH metabolites and lung function or FeNO in a large sample of U.S. children with and without asthma. Among children with asthma, we then tested whether vitamin D insufficiency modifies the estimated effect of urinary PAH metabolites on lung function.
METHODS
Subject recruitment
In this analysis, we included children (6-17 years old) who participated in the 2007-2008, 2009-2010, and 2011-2012 NHANES cycles with urinary PAH measurements. NHANES is a cross-sectional nationwide survey designed to assess the health and nutritional status of the noninstitutionalized U.S. population. Participants are selected by using a stratified multistage probability sampling, and oversampling persons 60 years and older and ethnic minorities (African Americans and Hispanics), to represent the U.S. population of all ages and produce reliable statistics. The survey combines interviews and physical examinations that are administered by highly trained personnel. NHANES is approved by the Institutional Review Board of the National Center for Health Statistics of the U.S. Centers for Disease Control and Prevention (CDC). Informed consent is obtained from all participants. A proxy provided information for survey participants who were less than 16 years of age and for subjects who could not answer the questions by themselves.
Study procedures
Current asthma was defined by a positive answer to both of the following questions: “Has a doctor or other health professional ever told you that you have asthma?”, and “Do you still have asthma?”. Participating children performed a pre-bronchodilator spirometry following American Thoracic Society recommendations18. Participants were not eligible for spirometry if they were on supplemental oxygen or had painful ear infections, current chest pain or a physical problem with forceful expiration, surgery (of the eye, chest, or the abdomen) in the previous 3 months, heart disease, history of a detached retina, hemoptysis, or history of a collapsed lung or tuberculosis exposure. The best FEV1, FVC, and the FEV1/FVC ratio were selected for analysis. Percent (%) predicted and z-score ofFEV1, FVC and FEV1/FVC were calculated using Global Lung Initiative equations that account for age, sex, race/ethnicity, and height19. FeNO), was measured using the Aerocrine NIOX MINO, a portable, hand-held NO analyzer (Aerocrine AB, Solna, Sweden). The NHANES protocol required two valid FeNO measurements that were reproducible. Body mass index (BMI) was calculated as weight in kilograms divided by height (in meters) squared based on body measures. BMI z scores were calculated based on the 2000 CDC growth charts20.
Laboratory procedures
Urinary PAH metabolites were examined in a randomly selected sample of subjects, comprising one-third of NHANES participants aged 6 years and older. Nine mono-hydroxylated metabolites of PAH (OH-PAH) in urine samples (including 1-hydroxynaphthalene, 2-hydroxynaphthalene, 2-hydroxyfluorene, 3-hydroxyfluorene, 9-hydroxyfluorene, 1-hydroxyphenanthrene, 2-hydroxyphenanthrene, 3-hydroxyphenanthrene, and 1-hydroxypyrene) were measured by isotope dilution capillary gas chromatography tandem mass spectrometry (GC-MS/MS). Analytes with values under the lower limit of detected (LLOD) were assigned a constant value [lower limit of detection divided by square root of 2 (LLOD/sqrt(2)]. Three variables were then created: the sum of individually calculated molar mass of all 9 PAH metabolites (ΣmolPAH), the sum of the naphthalene metabolites that have 2 benzene rings (Σmol2-PAH), and the sum of PAH metabolites that have 3 (fluorene and phenanthrene) or 4 (pyrene) benzene rings (Σmol3,4-PAH). Quartiles of ΣmolPAH, Σmol2-PAH, and Σmol3,4-PAH were categorized for the analysis of PAH metabolites and current asthma or lung function. Urinary creatinine was measured by the Roche/Hitachi Modular P Chemistry Analyzer to account for variation in dilution in spot urinary samples. Total serum 25(OH)D level was measured by ultra-high-performance liquid chromatography-tandem mass spectrometry (UHPLC-MS/MS). Serum total 25(OH)D (in SI units of nmol/L) was defined as the sum of 25(OH)D3 and 25(OH) D2, and then converted to conventional units (ng/mL), using the suggested conversion factors: 1 nmol/L=0.40066 ng/mL21. Vitamin D insufficiency was defined as a serum 25(OH)D level lower than 30 ng/ml. Further details of the methods, protocols, and definitions used in NHANES can be found at http://www.cdc.gov/nchs/nhanes.htm.
Because humans are often exposed to mixtures of the PAH compounds, sum of the individual PAH metabolites by chemical structures was calculated to estimate overall exposure.
Statistical analysis
Primary sampling units and strata for the complex NHANES survey design were applied for data analysis. Subsample weights, stratification, and clusters provided in the NHANES dataset were incorporated into the analysis to obtain proper estimates and their standard errors. Wald chi-square tests and t-tests were used for bivariate analyses of binary and continuous variables, respectively. Logistic regression was used for the analysis of urinary PAH metabolites and current asthma. Linear regression was used to examine urinary PAH metabolites and %predicted lung function or FeNO, separately in children with and without current asthma. All multivariable models were adjusted for age, gender, race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, or other), health insurance coverage, BMI z-score, serum cotinine (a marker of second-hand smoke exposure, SHS), urinary creatinine levels, and use of oral or inhaled steroids in the past 2 days. Family history of asthma was also included in the model for the analysis of PAH exposure and current asthma. We then tested for a multiplicative interaction between urinary PAH metabolites and vitamin D insufficiency on lung function in children with asthma. Since this interaction was statistically significant, we repeated the multivariable analysis after stratification by vitamin D insufficiency. All statistical analyses were conducted using the SAS SURVEY procedure and SAS 9.3 software (SAS Institute Inc., Cary, NC).
RESULTS
A total of 2,459 children who participated in NHANES (2007-2008, 2010-2011, and 2011-2012) and who had measures of urinary PAH, and data on asthma and lung function, were included in this analysis (Table 1). The overall prevalence of current asthma among study participants was 11.8%. Compared to children without asthma, those with asthma were more likely to be non-Hispanic black, and to have: health insurance, a higher BMI z score, vitamin D insufficiency, family history of asthma, a higher FeNO, used oral or inhaled steroid in the past 2 days, and lower %predicted FEV1 and lower %predicted FEV1/FVC. Compared with children without asthma, those with asthma had slightly higher levels of ΣmolPAH, Σmol2-PAH, or Σmol3,4-PAHs, but these differences were not statistically significant. With the exception of BMI z-score and urinary creatinine level, there were no significant differences in the main characteristics of children who were (n=2,459) and were not (n=6,346) included in the current analysis (E-Table 1).
Table 1-.
Characteristics of participating children (6-17 years) with and without asthma, NHANES 2007-2012
| Characteristics | No current asthma (n=2,170) |
With current asthma (n=289) |
|---|---|---|
| Age (years) | 11.5 ± 0.1 | 12.1 ± 0.3 |
| Male gender | 1106 (50.9) | 141 (49.8) |
| Race/ethnicity | ||
| Non-Hispanic white | 642 (56.7) | 86 (54.6)‡ |
| Non-Hispanic Black | 485 (13.2) | 103 (22.6) |
| Mexican American/other Hispanic | 865 (23.0) | 70 (13.9) |
| Other | <178 (7.1) | 30 (8.9) |
| Had health insurance coverage | 1418 (89.6) | 198 (95.1)‡ |
| Annual household income < $20,000 | 437 (14.5) | 81 (21.0) |
| Body mass index (BMI) z-score | 0.65 ± 0.03 | 0.90 ± 0.09‡ |
| Serum cotinine level (ng/ml) | 4.5 ± 0.9 | 6.9 ± 2.7 |
| Serum vitamin D level (ng/ml) | 27.5 ± 0.5 | 26.2 ± 1.0 |
| Vitamin D insufficiency (<30 ng/ml) | 1395 (62.7) | 205 (71.4)† |
| Family history of asthma | 561 (24.4) | 175 (59.0)‡ |
| Fractional exhaled nitric oxide (ppb) | 15.4 ± 0.5 | 23.2 ± 2.0‡ |
| Use oral or inhaled steroid in past 2 days | 13 (0.7) | 56 (18.4)‡ |
| %predicted FEV1 | 104.2 ± 0.4 | 99.2 ± 1.0‡ |
| %predicted FVC | 106.2 ± 0.5 | 105.2 ± 1.2 |
| %predicted FEV1/FVC | 97.7 ± 0.2 | 93.7 ± 0.6‡ |
| ΣPAHs (ng/L)a | 9861 ± 470 | 13322 ± 2880 |
| Σ2-PAHs (ng/L)b | 8474 ± 441 | 11717 ± 2837 |
| Σ3,4-PAHs (ng/L)c | 1371 ± 54 | 1512 ± 121 |
| Urinary creatinine (mg/dL) | 118.0 ± 2.3 | 132.6 ± 7.7 |
Results are shown as mean ± standard error (SE) for continuous variables, and as N (%) for binary variables. PAHs= polycyclic aromatic hydrocarbons
ΣPAHs = sum of all urinary PAH metabolites
Σ2-PAHs = sum of urinary PAHs with 2 benzene rings
Σ3,4-PAHs = sum of urinary PAHs with 3 or 4 benzene rings
P<0.05
P<0.01 for comparison between children with and without current asthma
The results of the multivariable analysis of urinary PAH metabolites (by quartiles) and current asthma are shown in Figure 1. After adjusting for age, gender, race/ethnicity, health insurance coverage, family history of asthma, serum cotinine and urinary creatinine, children in the highest quartile of urinary Σmol3,4-PAH had 2.84 times higher odds of current asthma than those in the lowest quartiles of urinary Σmol3,4-PAH (95% confidence interval [CI] for the odds ratio [OR] for 4th quartile vs. 1st quartile = 1.20 to 6.70, p=0.02). There was no significant association between either urinary ΣmolPAH or urinary Σmol2-PAH and current asthma.
Figure 1-.
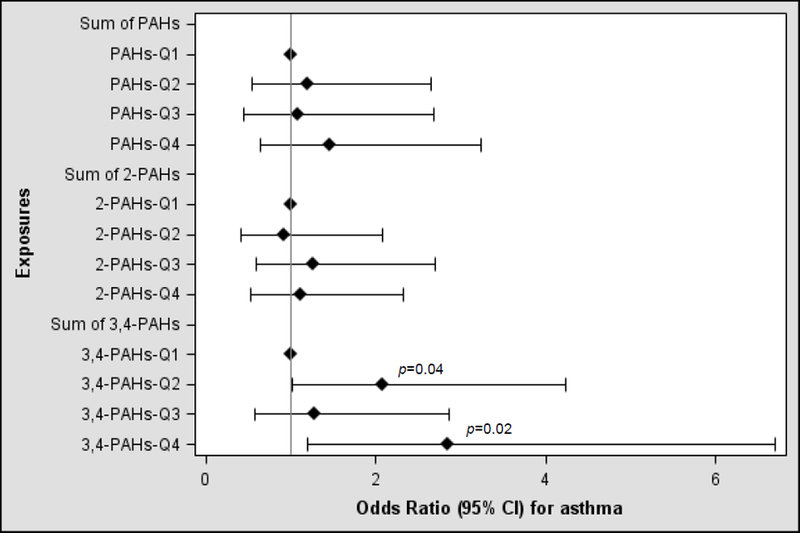
Urinary PAHs by quartiles (Q) and current asthma in children, NHANES 2007-2012
Since all analyses of urinary PAH metabolites (by quartiles) and lung function measures showed significant linear trends, continuous measures of urinary PAH metabolites were used in the analysis of lung function. Table 2 presents the results of the multivariable analyses of urinary PAH metabolites and lung function or FeNO, stratified by current asthma. Among children with asthma, each molar mass increase in ΣmolPAH was associated with 3.80% decrements in %predicted FEV1 (95% CI=−6.68 to −0.88) and each molar mass increase in Σmol2-PAH were associated with 3.47% decreased %predicted FEV1 (95% CI=−6.07 to −0.88). There was no significant association between urinary PAH and lung function among children without asthma, or with FeNO in children with or without asthma
Table 2-.
Urinary PAH and lung function (%predicted) and FeNO in children, by asthma status
| No current asthma (n=2,170) |
Current asthma (n=289) |
|
|---|---|---|
| Outcomes | β (95% confidence interval) | |
| ΣmolPAHsa | ||
| %predicted FEV1 | 0.94 (−0.31, 2.18) | −3.80 (−6.68, −0.88)† |
| %predicted FVC | 1.19 (−0.01, 2.36) | −2.13 (−5.12, 0.87) |
| %predicted FEV1/FVC | −0.10 (−0.84, 0.64) | −2.43 (−5.34, 0.49) |
| FeNO (ppb) | −0.57 (−2.01, 0.87) | −0.71 (−5.23, 3.80) |
| Σmol2-PAHsb | ||
| %predicted FEV1 | 0.67 (−0.50, 1.84) | −3.47 (−6.07, −0.88)† |
| %predicted FVC | 0.90 (−0.19, 2.00) | −2.20 (−4.79, 0.39) |
| %predicted FEV1/FVC | −0.09 (−0.80, 0.62) | −1.14 (−3.23, 0.96) |
| FeNO (ppb) | −0.44 (−1.81, 0.93) | −0.15 (−4.10, 3.79) |
| Σmol3,4-PAHsc | ||
| %predicted FEV1 | 0.59 (−1.33, 2.51) | −3.83 (−8.74, 1.09) |
| %predicted FVC | 0.42 (−1.37, 2.21) | −2.93 (−7.28, 1.42) |
| %predicted FEV1/FVC | 0.30 (−0.81, 1.42) | −1.27 (−4.33, 1.79) |
| FeNO (ppb) | −1.87 (−3.73, 0.01) | 2.12 (−3.98, 8.21) |
All models adjusting for health insurance coverage, BMI z-score, use of oral or inhaled steroids in the past 2 days, serum cotinine, and urinary creatinine level. Models for FeNO in additionally adjusting for age, gender, and race/ethnicity.
ΣmolPAHs = sum of all urinary PAH metabolites
Σmol2-PAHs = sum of urinary PAHs with 2 benzene rings
Σmol3,4-PAHs = sum of urinary PAHs with 3 or 4 benzene rings
P<0.05
Next, we tested for interactions between urinary PAH metabolites (as continuous) and vitamin D insufficiency (as binary) on lung function (%predicted FEV1, %predicted FVC, and %predicted FEV1/FVC) in children with asthma. Because we indeed found significant interactions between vitamin D insufficiency and urinary PAH metabolites in children with asthma (e.g., for ΣmolPAH, Σmol2-PAH, and Σmol3,4-PAH) on %predicted FEV1/FVC, P<0.01 in all instances), we repeated the multivariable analysis after stratification by vitamin D insufficiency. In this analysis, there was a significant inverse association between urinary PAH metabolites (ΣmolPAH, Σmol2-PAH, and Σmol3,4-PAH) and both %predicted FEV1 and %predicted FEV1/FVC in children with asthma and vitamin D insufficiency, but not in children with asthma and sufficient vitamin D levels (Table 3). Similar results were obtained in an analysis using z-scores for spirometry variables (E-Table 2)
Table 3-.
Urinary PAH and lung function (%predicted) in asthmatic children, by vitamin D status
| Vitamin D ≥ 30 ng/ml (n = 52) |
Vitamin D < 30 ng/ml (n = 205) |
|
|---|---|---|
| Outcomes | β (95% confidence interval) | |
| ΣmolPAHsa | ||
| %predicted FEV1 | 0.63 (−3.71, 4.97) | −5.13 (−8.60, −1.66)‡ |
| %predicted FVC | −1.44 (−6.25, 3.37) | −2.70 (−6.65, 1.25) |
| %predicted FEV1/FVC | 1.96 (−0.80, 4.72) | −2.33 (−4.26, −0.41)† |
| Σmol2-PAHsb | ||
| %predicted FEV1 | 0.60 (−2.77, 3.97) | −4.87 (−8.20, −1.54)‡ |
| %predicted FVC | −1.34 (−4.77, 2.09) | −2.72 (−6.47, 1.03) |
| %predicted FEV1/FVC | 1.86 (−0.70, 4.42) | −2.04 (−3.88, −0.19)† |
| Σmol3,4-PAHsc | ||
| %predicted FEV1 | −1.82 (−11.05, 7.41) | −5.57 (−11.12, −0.02)† |
| %predicted FVC | −0.89 (−11.23, 9.45) | −1.95 (−7.42, 3.52) |
| %predicted FEV1/FVC | −1.05 (−4.61, 2.50) | −3.94 (−6.39, −1.49)‡ |
All models adjusting for health insurance coverage, BMI z-score, use of oral or inhaled steroids in the past 2 days, serum cotinine, and urinary creatinine level.
ΣmolPAHs = sum of all urinary PAH metabolites
Σmol2-PAHs = sum of urinary PAHs with 2 benzene rings
Σmol3,4-PAHs = sum of urinary PAHs with 3 or 4 benzene rings
P<0.05,
P<0.01
DISCUSSION
In a large study of U.S. children, high levels of urinary Σmol3,4-PAH were associated with current asthma. Among children with asthma, urinary ΣmolPAH and Σmol2-PAH metabolites were associated with lower FEV1 and FVC. After stratification by vitamin D status, urinary ΣmolPAH, Σmol2-PAH and Σmol3,4-PAH were associated with lower FEV1 and FVC in children with asthma and vitamin D insufficiency, but not in those with asthma and sufficient vitamin D levels. Thus, our findings suggest potential synergistic detrimental effects of PAH exposure and vitamin D insufficiency on lung function in children with asthma.
PAH that have 3 or 4-rings (phenanthrene and pyrene) are dominant components of total PAH emission from diesel vehicles22 and coal combustion23. Our results for urinary Σmol3,4-PAH and childhood asthma are consistent with those of prior studies showing that urinary PAH metabolites such as phenanthrene are associated with asthma or wheeze in U.S. children9-11,24. Prenatal exposure to airborne PAH has been associated with increased benzo[a]pyrene-DNAs adducts, reduced expression of interferon-ɣ, and changes in DNA methylation in cord blood that have been ultimately linked to childhood asthma8,25,26. In addition, a combination of prenatal exposure to PAH and exposure to second-hand smoke or particulate matter has been linked to respiratory symptoms and probable asthma in young children27,28, and pyrene exposure during pregnancy has been associated with childhood asthma29. The association between Σmol3,4-PAH and childhood asthma was seen in the 2nd and 4th quartiles of Σmol3,4-PAH (but not 3rd quartile), suggesting a non-linear association.
In our study, urinary PAH metabolites were inversely associated with pre-bronchodilator FEV1 and FEV1/FVC among children with asthma and vitamin D insufficiency. In a prior study in California, the sum of PAH with 4, 5, or 6 rings was associated with decreased post-bronchodilator lung function in children without asthma, but not in children with asthma14. In further contrast to our results, PAH exposure was not associated with pre-bronchodilator lung function in children with or without asthma14. However, vitamin D status, use of oral or inhaled steroids, or exposure to second-hand smoke exposure were not assessed in that prior study, which also had a small sample size14.
Our finding of potential joint effects of vitamin D insufficiency and urinary PAH metabolites on lung function in asthmatic children is consistent with and expand those of our previous study in a Puerto Rican cohort, in whom residential proximity to a major highway (a surrogate marker of traffic-related air pollution) and vitamin D insufficiency conferred a higher risk of severe asthma exacerbations than having only one or none of these two risk factors17. Our results suggest that airway inflammation and oxidative stress induced by air pollutants is worsened by vitamin D insufficiency, as vitamin D has antioxidant effects30. In further support of this hypothesis, a longitudinal study showed that vitamin D deficiency enhanced the detrimental effects of smoking on lung function decline, indirectly suggesting that vitamin D may mitigate the damaging effects of smoking on lung function31. Alternatively, vitamin D insufficiency and PAH exposure may impair response to inhaled corticosteroids in children with asthma, ultimately leading to abnormal lung function30. In experimental studies, particular matter (which includes PAH as components) has been shown to promote T-helper 17 cell (Th17) responses in human myeloid dendritic cells, while vitamin D treatment attenuated expression of Th17 related cytokines and maintained IL-10 synthesis in dendritic cells treated with particular matter32.
Although we found no significant association between urinary PAH metabolites and FeNO (a marker of eosinophilic airway inflammation) in children with or without asthma, previous studies have shown that traffic exhaust contributes to allergic sensitization (not measured in the current study) and respiratory symptoms in children33, and that urinary phenanthrene and fluorene are linked to allergic sensitization to mouse in inner-city children34. Diesel exhaust particles that comprise PAHs have been shown to not only increase the effects of antigen exposure in those sensitized but also to promote antigen sensitization after allergen exposure35. Moreover, higher serum PAH have been positively correlated with serum total IgE, IL-4 and IL-5 in children with asthma36.
Our study has several strengths, including data on a large and ethnically diverse cohort of children, performance of standardized procedures by uniformly trained personnel, and the ability to account for several potential confounders. We also recognize several limitations to our findings. First, a temporal relationship between PAH exposure and asthma or lung function cannot be determined due to the cross-sectional study design. For example, children exposed to PAH through air pollution may reduce outdoor activities and thus have lower vitamin D levels. Second, while aggregating PAH metabolites allowed us to measure PAH from all routes of exposure, it also limited our ability to study specific exposures from inhalation or single PAH or separating the PAH sources (e.g. cigarette smoking or air pollution). Third, single urinary samples reflect recent exposures, and thus do not account for seasonal variability or long-term exposure to PAH. Lastly, the study did not account for several potential confounders, including outdoor activity, dietary factors, or exposure to other air pollutants.
In summary, exposure to PAH was associated with worse lung function in U.S. children with asthma, particularly in those with vitamin D insufficiency. Ongoing clinical trials should help determine whether vitamin D supplementation ameliorates the detrimental effects of air pollution on lung function and disease severity among children with asthma.
Supplementary Material
Acknowledgments
Sources of Funding: Dr. Forno’s contribution was supported by grants from the U.S. National Institutes of Health (NIH, HL125666), Children’s Hospital of Pittsburgh, and the Klosterfrau Foundation. Dr. Celedón’s contribution was supported by grants from the U.S. NIH (HL117191, HL119952, and MD011764).
Footnotes
Footnote:
All models adjusting for age, gender, race/ethnicity, health insurance coverage, family history of asthma, serum cotinine, and urinary creatinine level. PAH levels were calculated as molecular weight/L.
References
- 1.Ferkol T, Schraufnagel D. The global burden of respiratory disease. Annals of the American Thoracic Society. 2014;11(3):404–406. [DOI] [PubMed] [Google Scholar]
- 2.Eder W, Ege MJ, von Mutius E. The asthma epidemic. The New England journal of medicine. 2006;355(21):2226–2235. [DOI] [PubMed] [Google Scholar]
- 3.Guarnieri M, Balmes JR. Outdoor air pollution and asthma. Lancet. 2014;383(9928):1581–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karimi P, Peters KO, Bidad K, Strickland PT. Polycyclic aromatic hydrocarbons and childhood asthma. European journal of epidemiology. 2015;30(2):91–101. [DOI] [PubMed] [Google Scholar]
- 5.Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological profile for Polycyclic Aromatic Hydrocarbons (PAHs). In: U.S. Department of Health and Human Services PHS, ed. Atlanta, GA: 1995. [PubMed] [Google Scholar]
- 6.Strickland P, Kang D, Sithisarankul P. Polycyclic aromatic hydrocarbon metabolites in urine as biomarkers of exposure and effect. Environmental health perspectives. 1996;104 Suppl 5:927–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Preuss R, Angerer J, Drexler H. Naphthalene--an environmental and occupational toxicant. International archives of occupational and environmental health. 2003;76(8):556–576. [DOI] [PubMed] [Google Scholar]
- 8.Perera F, Tang WY, Herbstman J, et al. Relation of DNA methylation of 5’-CpG island of ACSL3 to transplacental exposure to airborne polycyclic aromatic hydrocarbons and childhood asthma. PloS one. 2009;4(2):e4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gale SL, Noth EM, Mann J, Balmes J, Hammond SK, Tager IB. Polycyclic aromatic hydrocarbon exposure and wheeze in a cohort of children with asthma in Fresno, CA. Journal of exposure science & environmental epidemiology. 2012;22(4):386–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu H, Xu C, Jiang ZY, Gu A . Association of polycyclic aromatic hydrocarbons and asthma among children 6-19 years: NHANES 2001-2008 and NHANES 2011-2012. Respiratory medicine. 2016;110:20–27. [DOI] [PubMed] [Google Scholar]
- 11.Barraza-Villarreal A, Escamilla-Nunez MC, Schilmann A, et al. Lung function, airway inflammation, and polycyclic aromatic hydrocarbons exposure in mexican schoolchildren: a pilot study. Journal of occupational and environmental medicine. 2014;56(4):415–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cakmak S, Hebbern C, Cakmak JD, Dales RE. The influence of polycyclic aromatic hydrocarbons on lung function in a representative sample of the Canadian population. Environ Pollut. 2017;228:1–7. [DOI] [PubMed] [Google Scholar]
- 13.Zhou Y, Sun H, Xie J, et al. Urinary Polycyclic Aromatic Hydrocarbon Metabolites and Altered Lung Function in Wuhan, China. American journal of respiratory and critical care medicine. 2016;193(8):835–846. [DOI] [PubMed] [Google Scholar]
- 14.Padula AM, Balmes JR, Eisen EA, et al. Ambient polycyclic aromatic hydrocarbons and pulmonary function in children. Journal of exposure science & environmental epidemiology. 2015;25(3):295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klingbeil EC, Hew KM, Nygaard UC, Nadeau KC. Polycyclic aromatic hydrocarbons, tobacco smoke, and epigenetic remodeling in asthma. Immunologic research. 2014;58(2-3):369–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han YY, Forno E, Celedon JC. Vitamin D Insufficiency and Asthma in a US Nationwide Study. The journal of allergy and clinical immunology In practice. 2017;5(3):790–796 e791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosser F, Brehm JM, Forno E, et al. Proximity to a major road, vitamin D insufficiency, and severe asthma exacerbations in Puerto Rican children. American journal of respiratory and critical care medicine. 2014;190(10):1190–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Standardization of Spirometry, 1994 Update. American Thoracic Society. American journal of respiratory and critical care medicine. 1995;152(3):1107–1136. [DOI] [PubMed] [Google Scholar]
- 19.Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. The European respiratory journal. 2012;40(6):1324–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention National Center for Health Statistics. 2000 CDC Growth Charts for the United States: Methods and Development. In: Department of Health and Human Services, ed. Vol 11. Hyattsville, Maryland: 2002. [Google Scholar]
- 21.Center for Disease Control and Prevention. Analytical Note for 25-Hydroxyvitamin D Data Analysis using NHANES III (1988-1994), NHANES 2001-2006, and NHANES 2007-2010 2015; http://wwwn.cdc.gov/Nchs/Nhanes/VitaminD/AnalyticalNote.aspx?h=https://wwwn.cdc.gov/Nchs/Nhanes/2009-2010/VID_F.htm&t=VID_F%20Doc. Accessed April 21, 2016.
- 22.Zheng X, Wu Y, Zhang S, et al. Characterizing particulate polycyclic aromatic hydrocarbon emissions from diesel vehicles using a portable emissions measurement system. Scientific reports. 2017;7(1):10058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen G, Wang W, Yang Y, et al. Emission factors and particulate matter size distribution of polycyclic aromatic hydrocarbons from residential coal combustions in rural Northern China. Atmos Environ (1994). 2010;44(39):5737–5743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jung KH, Perzanowski M, Rundle A, et al. Polycyclic aromatic hydrocarbon exposure, obesity and childhood asthma in an urban cohort. Environmental research. 2014;128:35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herbstman JB, Tang D, Zhu D, et al. Prenatal exposure to polycyclic aromatic hydrocarbons, benzo[a]pyrene-DNA adducts, and genomic DNA methylation in cord blood. Environmental health perspectives. 2012;120(5):733–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang WY, Levin L, Talaska G, et al. Maternal exposure to polycyclic aromatic hydrocarbons and 5’-CpG methylation of interferon-gamma in cord white blood cells. Environmental health perspectives. 2012;120(8):1195–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller RL, Garfinkel R, Horton M, et al. Polycyclic aromatic hydrocarbons, environmental tobacco smoke, and respiratory symptoms in an inner-city birth cohort. Chest. 2004;126(4):1071–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jedrychowski WA, Perera FP, Maugeri U, et al. Intrauterine exposure to polycyclic aromatic hydrocarbons, fine particulate matter and early wheeze. Prospective birth cohort study in 4-year olds. Pediatric allergy and immunology : official publication of the European Society of Pediatric Allergy and Immunology. 2010;21(4 Pt 2):e723–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jung KH, Yan B, Moors K, et al. Repeated exposure to polycyclic aromatic hydrocarbons and asthma: effect of seroatopy. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2012;109(4):249–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paul G, Brehm JM, Alcorn JF, Holguin F, Aujla SJ, Celedon JC. Vitamin D and asthma. American journal of respiratory and critical care medicine. 2012;185(2):124–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lange NE, Sparrow D, Vokonas P, Litonjua AA. Vitamin D deficiency, smoking, and lung function in the Normative Aging Study. American journal of respiratory and critical care medicine. 2012;186(7):616–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mann EH, Ho TR, Pfeffer PE, et al. Vitamin D Counteracts an IL-23-Dependent IL-17A(+)IFN-gamma(+) Response Driven by Urban Particulate Matter. American journal of respiratory cell and molecular biology. 2017;57(3):355–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Braback L, Forsberg B. Does traffic exhaust contribute to the development of asthma and allergic sensitization in children: findings from recent cohort studies. Environmental health : a global access science source. 2009;8:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller RL, Garfinkel R, Lendor C, et al. Polycyclic aromatic hydrocarbon metabolite levels and pediatric allergy and asthma in an inner-city cohort. Pediatric allergy and immunology : official publication of the European Society of Pediatric Allergy and Immunology. 2010;21(2 Pt 1):260–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Diaz-Sanchez D, Garcia MP, Wang M, Jyrala M, Saxon A. Nasal challenge with diesel exhaust particles can induce sensitization to a neoallergen in the human mucosa. The Journal of allergy and clinical immunology. 1999;104(6):1183–1188. [DOI] [PubMed] [Google Scholar]
- 36.Al-Daghri NM, Alokail MS, Abd-Alrahman SH, Draz HM, Yakout SM, Clerici M. Polycyclic aromatic hydrocarbon exposure and pediatric asthma in children: a case-control study. Environmental health : a global access science source. 2013;12:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


