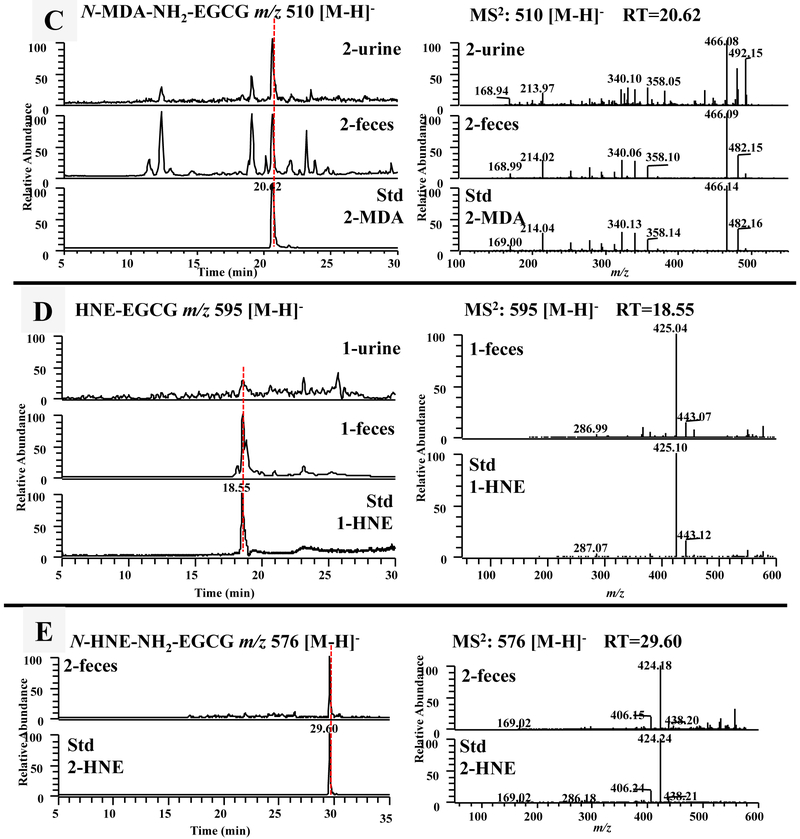
Figure 5.
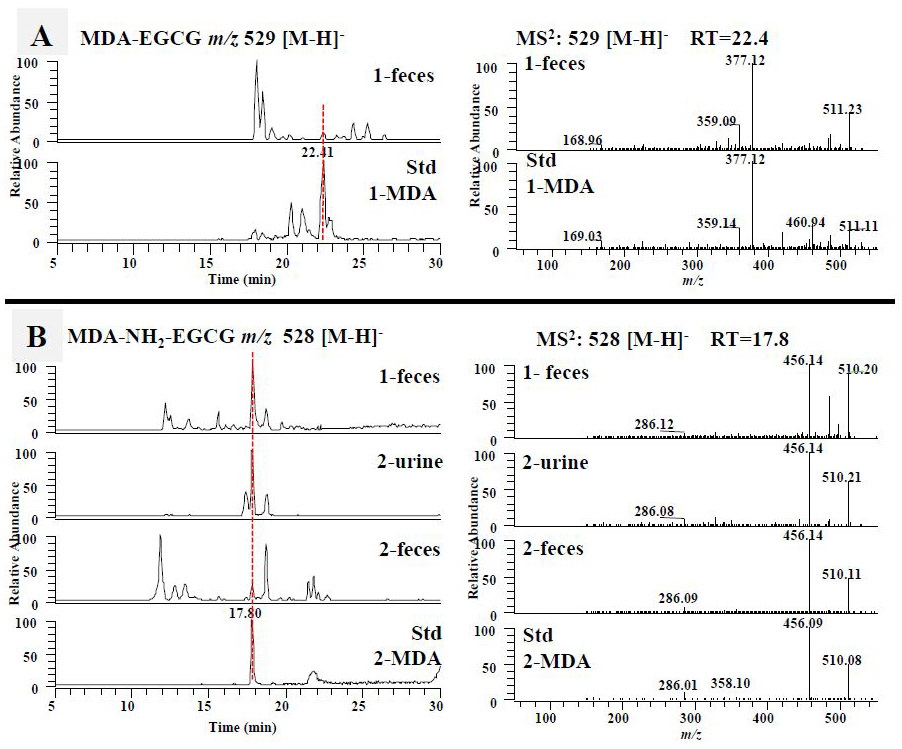
The formation of the malondialdehyde (MDA) and 4-hydroxy-trans-2-nonenal (4-HNE) conjugated metabolites of EGCG and 4'-NH2-EGCG in mice. (A) The LC chromatograms and the ESI-MS2 spectra of mono MDA-EGCG (m/z 529 [M-H]−) of mouse fecal samples collected from EGCG (1) treated mice and the sample collected from chemical incubation of EGCG (1) with MDA in phosphate buffer (pH 7.4) at 37 °C for 6 h. The LC chromatograms and the ESI-MS2 spectra of MDA-NH2-EGCG (m/z 528 [M-H]−) (B) and N-MDA-4'-NH2-EGCG (m/z 510 [M-H]−) (C) of mouse fecal and urinary samples collected from EGCG (1) treated mice, 4'-NH2-EGCG (2) treated mice, and the sample collected from chemical incubation of 4'-NH2-EGCG (2) with MDA in phosphate buffer (pH 7.4) at 37 °C for 6 h. (D) The LC chromatograms and the ESI-MS2 spectra of mono HNE-EGCG (m/z 595 [M-H]−) of mouse urinary and fecal samples collected from EGCG (1) treated mice and the sample collected from chemical incubation of EGCG (1) with HNE in phosphate buffer (pH 7.4) at 37 °C for 6 h. (E) The LC chromatograms and the ESI-MS2 spectra of N-HNE-4'-NH2-EGCG (m/z 576 [M-H]−) of mouse fecal samples collected from 4'-NH2-EGCG (2) treated mice and the sample collected from chemical incubation of 4'-NH2-EGCG (2) with HNE in phosphate buffer (pH 7.4) at 37 °C for 6 h.