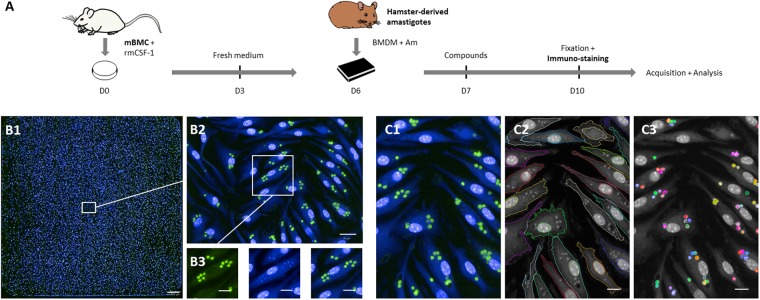
Figure 1.
Schematic overview of the L. donovani screening assay. (A) High Content Assay (HCA) pipeline. Mouse bone marrow cells (mBMC) were cultured in presence of rmCSF-1 for 6 days before plating differentiated BMDMs in 384-well plates. Five and 20 hours later, hamster-derived amastigotes (Am) and compounds were added to BMDM cultures, respectively. After 3 days of incubation, BMDMs were fixed and subjected to immuno-staining followed by image acquisition and analysis. (B1–3) HCA readout. Image acquisition was performed with the OPERA QEHS High Content Imaging System using the following acquisition settings: 488 nm laser line for excitation and filter 540/75 for immuno-stained parasites; 405 nm laser line for excitation and filter 450/50 for Hoechst-stained nuclei and HCS CellMask cytoplasm detection. (B1) Merged fluorescence images acquired with a 40x water immersion objective (NA 1.5) showing the 285 fields of an entire well initially seeded with 20,000 BMDMs and inoculated with Ld1S amastigotes (MOI of 6). Scale bar, 200 µm. (B2) Zoomed image corresponding to one acquisition field. Scale bar, 20 µm. (B3) Cropped and zoomed image showing fluorescent staining at the cellular level. Left panel, immunostained amastigotes in green; middle panel, Hoechst 33342 and HCS CellMask staining in blue; right panel, merged images. Scale bars, 10 µm. (C1–3) Illustration of object segmentation pipeline implemented in the Columbus platform: initial overlay image (C1), macrophage cell and nucleus segmentation outline (C2) and intracellular parasites (C3). Scale bars, 10 µm.