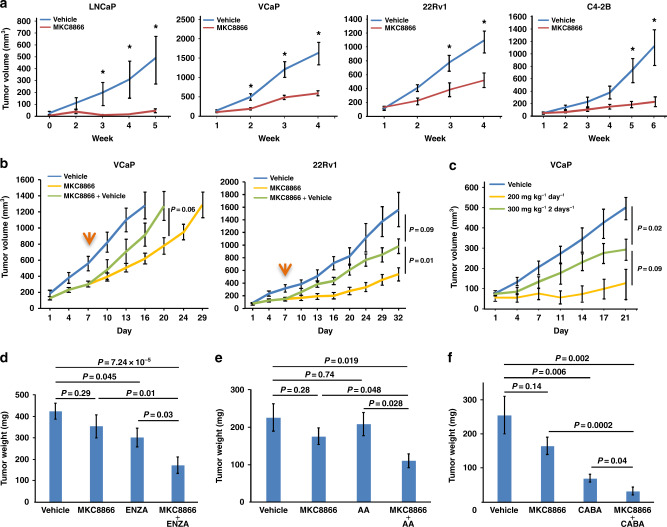
Fig. 2.
Therapeutic efficacy of MKC8866 in preclinical mouse models of PCa. a Nude mice bearing LNCaP, VCaP, 22Rv1, or C4-2B xenografts were treated orally with either vehicle or 300 mg kg−1 MKC8866 daily and tumor growth was recorded. b Nude mice bearing VCaP or 22Rv1 tumors were treated with either vehicle or 300 mg kg−1 MKC8866 daily. At day 7 (indicated by the orange arrow), the MKC8866-treated mice were randomly divided into two groups, one of which continuously received MKC8866 of the same dosage while the other group was treated with vehicle for the rest of the experiment. c Nude mice bearing VCaP xenografts were orally treated with either vehicle, 200 mg kg−1 MKC8866 daily, or 300 mg kg−1 MKC8866 every other day. Tumor growth was recorded weekly. d Nude mice bearing VCaP xenografts were orally treated either with vehicle, MKC8866 (300 mg kg−1 every two days), enzalutamide (ENZA, 30 mg kg−1 every two days), or MKC8866+ENZA. Tumor weight was recorded at the end of the experiment. e As in d, but the treatments were vehicle, MKC8866 (300 mg kg−1 every two days), abiraterone acetate (AA, 20 mg kg−1 every two days), or a combination of both drugs. f As in d, but the treatments were vehicle, or MKC8866 (300 mg kg−1 every two days), or cabazitaxel (CABA, 5 mg kg−1) twice a week intraperitoneally, or a combination of both drugs. *P < 0.05, all P values were calculated by Student’s t-test, error bars denote the SEM