Abstract
Ginseng, Panax ginseng C.A. Meyer, is one of the most important medicinal herbs for human health and medicine in which ginsenosides are known to play critical roles. The genes from the cytochrome P450 (CYP) gene superfamily have been shown to play important roles in ginsenoside biosynthesis. Here we report genome-wide identification of the candidate PgCYP genes for ginsenoside biosynthesis, development of functional SNP markers for its manipulation and systems analysis of its underlying molecular mechanism. Correlation analysis identified 100 PgCYP genes, including all three published ginsenoside biosynthesis PgCYP genes, whose expressions were significantly correlated with the ginsenoside contents. Mutation association analysis identified that six of these 100 PgCYP genes contained SNPs/InDels that were significantly associated with ginsenosides biosynthesis (P ≤ 1.0e-04). These six PgCYP genes, along with all ten published ginsenoside biosynthesis genes from the PgCYP and other gene families, formed a strong co-expression network, even though they varied greatly in spatio-temporal expressions. Therefore, this study has identified six new ginsenoside biosynthesis candidate genes, provided a genome-wide insight into how they are involved in ginsenoside biosynthesis and developed a set of functional SNP markers useful for enhanced ginsenoside biosynthesis research and breeding in ginseng and related species.
Introduction
Ginseng (Panax ginseng C.A. Meyer) is one of the most important medicinal herbs for human health and medicine. Ginseng has been shown to have several bioactive components, including triterpene saponins, flavonoids, fructoligosaccharides and polysaccharides1, but the most valuable is ginsenosides, a diverse group of triterpene saponins that are produced in all tissues of ginseng2. Studies have shown that ginsenosides provide several benefits for human health, including immunomodulation3, anti-inflammation4, anti-tumor5,6, anti-hyperlipidemia7 and anti-amnestic and anti-aging activities8.
Therefore, isolation and characterization of the genes involved in the ginsenoside biosynthesis have been a major focus of ginseng research and breeding. However, only a few genes involved in this process have been thus far cloned, no molecular tools have been developed to manipulate the process for enhanced research and breeding, and little is known about the molecular mechanism underlying ginsenoside biosynthesis. The genes by far cloned that are involved in ginsenoside biosynthesis include those encoding squalene synthase (SS)9, cycloartenol synthase (CAS)10, squalene epoxidase (SE)11, dammarenediol synthase (DS)12,13, beta-amyrin synthase (β-AS)14, farnesyl phosphate synthase (FPS)15, UDP-glycosyltransferase (UGT)16, and cytochrome P450 (CYP716A53v2, CYP716A52v2 and CYP716A47)17–19. These studies indicate that it is likely that additional genes, including those of the CYP gene superfamily encoding cytochrome P450, are involved in ginsenoside biosynthesis.
The cytochrome P450, CYP450, gene superfamily is known to be widely present in all organisms, including plants, animals and microbes20. They encode monooxygenases involved in endogenous oxidation reaction, such as lipids, steroidal hormones and cholesterol as well as in the degradation of xenobiotics such as insecticide and herbicide21–25. The CYP450 genes also extensively participate in the modification of various secondary metabolites that potentially have pharmacological properties as well, endowing medicinal herbs with potential medical and economical values26. Over the last decades, the functions of the CYP450 genes have been studied in the biosynthesis of various herbal components, such as ginsenosides17–19, artemisinin27,28, nootkatone29, olean-12-ene-3β-24-diol soyasapogenol B30, oleanolic acid31, flavonones32 and hyoscyamine33.
In this study, we identified 100 CYP450 genes from ginseng, defined PgCYP genes, whose expressions were significantly correlated with variation of nine mono- and total-ginsenoside contents. We analyzed these 100 PgCYP genes by association study and identified five SNPs and three InDels from 6 of them that were significantly associated with the ginsenoside contents in the four-year-old roots of 42 genotypes. Finally, we characterized these six PgCYP genes in several aspects and examined their relationships with those published ginsenoside biosynthesis genes that were previously cloned from the PgCYP gene superfamily, CYP716A53v2, CYP716A52v2 and CYP716A4717–19, and from other gene families, including SS9, CAS10, SE11, DS12,13, β-AS14, FPS15 and UGT16. Therefore, this study has identified six new candidate PgCYP genes involved in ginsenoside biosynthesis and developed functional SNP/InDel markers for these genes, which are important for advanced ginsenoside biosynthesis research and enhanced breeding in ginseng and related species.
Results
Identification of the candidate PgCYP genes involved in ginsenoside biosynthesis
Han et al.17–19 cloned three CYP genes from P. ginseng, defined CYP716A53v2, CYP716A52v2 and CYP716A47, and showed that they were involved in ginsenoside biosynthesis by gene expression repression analysis using RNA interference (RNAi) technology. These results indicated that the PgCYP gene superfamily plays important roles in ginsenoside biosynthesis. Moreover, Syed et al.34 documented that most of the genes contained in a genome are subjected to RNA alternative splicing that often results in multiple transcripts from a single gene. These multiple transcripts are translated into different proteins having different biological functions. Therefore, we performed correlation analysis of the expression activities of all 440 PgCYP transcripts derived from 414 PgCYP genes that we previously identified35 with the variations of nine mono- and total-ginsenoside contents in the four-year-old roots of 42 cultivars to examine whether any of them is likely also involved in ginsenoside biosynthesis, in addition to CYP716A53v2, CYP716A52v2 and CYP716A4717–19.
We hypothesized if a gene is involved in ginsenoside biosynthesis, its expression activity should be correlated or associated with ginsenoside contents in ginseng. To test this hypothesis, we first conducted correlation analysis of the genes previously cloned from ginseng that were shown to be involved in the ginsenoside biosynthesis between their expressions and the ginsenoside contents in the four-year-old roots of 42 ginseng cultivars. These previously cloned ginsenoside biosynthesis genes included SS, CAS, SE, DS, β-AS, FPS, UGT and three CYP genes (CYP716A53v2, CYP716A52v2 and CYP716A47) (Supplementary Table S1). We extracted the expressions of every transcript of these ginsenoside biosynthesis genes from the root transcriptome database of the 42 ginseng cultivars (Supplementary Table S2)36 and conducted correlation analysis with the nine mono- and total-ginsenoside contents (Supplementary Table S3). The result showed that the expressions of 1–4 transcripts of each of the ten published ginsenoside biosynthesis genes were correlated with the variations of one or more of the mono- and total-ginsenoside contents at a significance level of P ≤ 0.05. When a significance level of P ≤ 0.01 was applied, the expressions of one or two transcripts of each of these ten published ginsenoside biosynthesis genes remained correlated with the variations of one or more of the mono- and total-ginsenoside contents (Supplementary Table S4). These results not only further verified the roles of these published genes in ginsenoside biosynthesis, but importantly, it also suggested that if a gene was significantly correlated in expression with the variations of the mono- and total-ginsenoside contents, it was highly likely, if not it was, involved in ginsenoside biosynthesis.
Therefore, we conducted the expression correlation analysis of all the 440 PgCYP transcripts with the variations of the nine mono- and total-ginsenoside contents in the four-year-old roots of the 42 cultivars. As a result, a total of 111 transcripts derived from 100 PgCYP genes were correlated in expression with the variations of the nine mono- and total-ginsenoside contents, when a significance level of P ≤ 0.05 was applied (Table 1; Supplementary Tables S5 and S6). For the nine mono-ginsenosides, the expressions of 9–46 transcripts per mono-ginsenoside were correlated with the variations of their contents in the four-year-old roots of 42 cultivars. For the total ginsenoside, the expressions of 48 transcripts were correlated with the variations of its content. When a significance level of P ≤ 0.01 was applied, a total of 62 transcripts derived from 59 PgCYP genes were correlated in expression with the variations of also all nine mono- and total-ginsenoside contents. For the nine mono-ginsenosides, the expressions of 3–18 transcripts per mono-ginsenoside were correlated with the variations of their contents. For the total ginsenoside, the expressions of 24 transcripts were correlated with the variations of its content. These results suggested that at least 100 gene members of the PgCYP gene superfamily are likely involved in ginsenoside biosynthesis in the four-year-old roots of the 42 cultivars at a confidence of >95%.
Table 1.
The PgCYP genes that are significantly correlated with the variation of mono- and total-ginsenoside contents in the four-year-old roots of 42 cultivars.
| Rg1 | Re | Rf | Rb1 | Rg2 | Rc | Rb2 | Rb3 | Rd | TS |
|---|---|---|---|---|---|---|---|---|---|
| PgCYP020 | PgCYP056 | PgCYP144 | PgCYP001 | PgCYP100 | PgCYP126 | PgCYP070 | PgCYP090 | PgCYP001 | PgCYP001 |
| PgCYP070 | PgCYP096 | PgCYP200 | PgCYP014 | PgCYP106 | PgCYP149 | PgCYP114 | PgCYP138 | PgCYP020 | PgCYP014 |
| PgCYP071 | PgCYP128 | PgCYP205 | PgCYP020 | PgCYP126 | PgCYP234 | PgCYP138 | PgCYP168 | PgCYP025 | PgCYP020 |
| PgCYP137 | PgCYP138 | PgCYP220 | PgCYP056 | PgCYP171 | PgCYP247 | PgCYP176 | PgCYP176 | PgCYP070 | PgCYP056 |
| PgCYP144 | PgCYP176 | PgCYP222 | PgCYP070 | PgCYP250 | PgCYP338 | PgCYP177 | PgCYP186 | PgCYP136 | PgCYP070 |
| PgCYP161 | PgCYP177 | PgCYP227 | PgCYP096 | PgCYP274-1 | PgCYP347-2 | PgCYP187 | PgCYP187 | PgCYP137 | PgCYP136 |
| PgCYP167 | PgCYP187 | PgCYP240 | PgCYP136 | PgCYP279 | PgCYP347-4 | PgCYP188 | PgCYP188 | PgCYP171 | PgCYP137 |
| PgCYP191 | PgCYP188 | PgCYP247 | PgCYP137 | PgCYP310-2 | PgCYP359-2 | PgCYP200 | PgCYP218 | PgCYP173 | PgCYP173 |
| PgCYP210 | PgCYP196 | PgCYP251 | PgCYP173 | PgCYP335-1 | PgCYP376-3 | PgCYP205 | PgCYP237 | PgCYP177 | PgCYP176 |
| PgCYP227 | PgCYP198 | PgCYP292 | PgCYP177 | PgCYP385 | PgCYP218 | PgCYP239 | PgCYP188 | PgCYP177 | |
| PgCYP301 | PgCYP205 | PgCYP339 | PgCYP188 | PgCYP396 | PgCYP237 | PgCYP278 | PgCYP205 | PgCYP188 | |
| PgCYP309 | PgCYP218 | PgCYP347-2 | PgCYP205 | PgCYP239 | PgCYP302 | PgCYP210 | PgCYP205 | ||
| PgCYP319 | PgCYP237 | PgCYP359-1 | PgCYP211 | PgCYP247 | PgCYP303 | PgCYP225 | PgCYP218 | ||
| PgCYP332 | PgCYP239 | PgCYP396 | PgCYP218 | PgCYP267 | PgCYP315-1 | PgCYP233 | PgCYP225 | ||
| PgCYP366 | PgCYP247 | PgCYP225 | PgCYP278 | PgCYP326 | PgCYP234 | PgCYP233 | |||
| PgCYP374 | PgCYP260 | PgCYP233 | PgCYP291 | PgCYP348-1 | PgCYP247 | PgCYP234 | |||
| PgCYP376-1 | PgCYP267 | PgCYP234 | PgCYP292 | PgCYP376-1 | PgCYP258 | PgCYP237 | |||
| PgCYP376-4 | PgCYP274-1 | PgCYP247 | PgCYP311 | PgCYP376-3 | PgCYP261-2 | PgCYP239 | |||
| PgCYP278 | PgCYP258 | PgCYP313-2 | PgCYP376-5 | PgCYP263 | PgCYP247 | ||||
| PgCYP285-1 | PgCYP261-2 | PgCYP326 | PgCYP378 | PgCYP264 | PgCYP258 | ||||
| PgCYP292 | PgCYP263 | PgCYP335-2 | PgCYP267 | PgCYP261-2 | |||||
| PgCYP311 | PgCYP264 | PgCYP348-1 | PgCYP269 | PgCYP263 | |||||
| PgCYP313-1 | PgCYP269 | PgCYP376-1 | PgCYP274-1 | PgCYP264 | |||||
| PgCYP313-2 | PgCYP272 | PgCYP376-5 | PgCYP274-2 | PgCYP267 | |||||
| PgCYP326 | PgCYP278 | PgCYP282 | PgCYP269 | ||||||
| PgCYP339 | PgCYP280 | PgCYP294 | PgCYP274-1 | ||||||
| PgCYP348-1 | PgCYP282 | PgCYP300 | PgCYP274-2 | ||||||
| PgCYP361 | PgCYP285-1 | PgCYP305 | PgCYP278 | ||||||
| PgCYP369 | PgCYP292 | PgCYP309 | PgCYP285-1 | ||||||
| PgCYP376-1 | PgCYP294 | PgCYP310-1 | PgCYP292 | ||||||
| PgCYP376-5 | PgCYP303 | PgCYP311 | PgCYP294 | ||||||
| PgCYP305 | PgCYP336 | PgCYP300 | |||||||
| PgCYP307 | PgCYP348-2 | PgCYP303 | |||||||
| PgCYP309 | PgCYP363-1 | PgCYP305 | |||||||
| PgCYP311 | PgCYP369 | PgCYP309 | |||||||
| PgCYP336 | PgCYP310-1 | ||||||||
| PgCYP339 | PgCYP311 | ||||||||
| PgCYP341 | PgCYP313-2 | ||||||||
| PgCYP342 | PgCYP326 | ||||||||
| PgCYP348-2 | PgCYP339 | ||||||||
| PgCYP361 | PgCYP341 | ||||||||
| PgCYP363-1 | PgCYP342 | ||||||||
| PgCYP363-2 | PgCYP347-2 | ||||||||
| PgCYP365 | PgCYP348-1 | ||||||||
| PgCYP369 | PgCYP348-2 | ||||||||
| PgCYP412 | PgCYP361 | ||||||||
| PgCYP363-1 | |||||||||
| PgCYP369 | |||||||||
| 18 | 31 | 14 | 46 | 9 | 11 | 24 | 20 | 35 | 48 |
Mono-ginsenosides, Rg1, Re, Rf, Rb1, Rg2, Rc, Rb2, Rb3 and Rd; Total ginsenosides, TS. For detail, see Supplementary Table S5.
Association study of the candidate PgCYP genes involved in ginsenoside biosynthesis
Furthermore, we carried out mutation analysis of these 100 PgCYP genes that were significantly correlated in expression with the variation of ginsenoside contents among the 42 cultivars. The single marker analysis method of QTL mapping37 and the candidate gene approach of genome-wide association study38 were used for the analysis. Because gene SNP/InDel mutation analysis has been widely used for gene functional analysis, this analysis would identify the genic or functional SNP/InDel markers for these 100 PgCYP genes and also provide an additional line of evidence on their functionality in ginsenoside biosynthesis. We identified a total of 724 SNPs/InDels from these 100 PgCYP genes. Association analysis showed that 78 of the 724 SNPs/InDels (10.8%) that were contained in 18 of the 100 PgCYP genes were associated with the variation of ginsenoside contents, when a significance level of P ≤ 0.05 was applied. These 18 PgCYP genes included all three PgCYP genes, CYP716A47, CYP716A53v2 and CYP716A52v2 previously cloned by Han et al.17–19. The reason that the remaining 646 SNPs were not associated with the variation of ginsenoside contents was because they were synonymous nucleotide substitutions that highly likely had no biological effects as they did not change the coding protein sequences39. Therefore, these 78 SNP/InDel mutations had significant influences on the contents of all nine mono- and total-ginsenosides and different mutations of a PgCYP gene might influence different ginsenosides. When a significance level of P ≤ 1.0E-04 (according to the Bonferroni multiple testing correction, P = 0.05/100 = 5.0E-04) was applied, the five SNPs and three InDels contained in six of the 18 PgCYP genes remained significantly associated with the variations of the ginsenosides (Table 2). Therefore, these six PgCYP genes were considered to be involved in the ginsenoside biosynthesis or to be the candidate genes involved in the ginsenoside biosynthesis. Of the eight SNP or InDel mutations, three (37.5%) were non-synonymous, one (12.5%) was synonymous, three (37.5%) were ORF-shifted, and one (12.5%) was not found in ORF. According to Graur and Li39, a vast majority of synonymous substitutions have no biological impacts, but recent studies showed that some of the synonymous SNPs or nucleotide substitutions may have biological functions40.
Table 2.
The PgCYP genes that are significantly correlated with the variation of ginsenoside contents in the four-year-old roots of 42 farmers’ cultivars and also have mutations that are significantly associated with ginsenoside contents.
| Gene | Position nucleotide mutation | Mutation type | Effect % (P-value) | ||||
|---|---|---|---|---|---|---|---|
| Rg1 | Re | Rc | Rb2 | TS | |||
| PgCYP149 | 70_CT_C | ORF shift | |||||
| 73_C_CGTGTGA AAATAAAAGAA ACCCAGAACCCA TTAAAGCACCAG | ORF shift | 52.0 (5.50E-04) | 30.7 (9.38E-05) | ||||
| PgCYP218 | 667_A_G | S | 69.08 (2.90E-05) | ||||
| 681_T_C | NS | 63.81 (3.60E-05) | |||||
| PgCYP222 | 647_T_G | NS | 62.7 (1.63E-04) | ||||
| PgCYP305 | 1214_A_AAT | ORF shift | 69.9 (1.19E-04) | ||||
| PgCYP341 | 140_G_A | Not in ORF | 73.1 (8.69E-04) | ||||
| PgCYP385 | 468_G_A | NS | 52.6 (8.08E-04) | ||||
Mono-ginsenosides, Rg1, Re, Rc and Rb2; Total ginosenosides, TS; S, synonymous substitution; NS, non-synonymous substitution; ORF, open-reading frame.
Characterization of the candidate PgCYP genes involved in ginsenoside biosynthesis
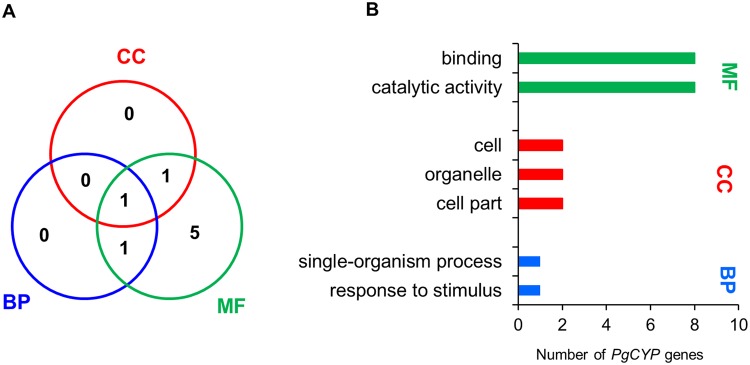
Because the SNP or InDel mutations of six of the 100 PgCYP genes that were significantly correlated with the variations of ginsenoside contents were also associated with ginsenoside biosynthesis at P ≤ 1.0E-04 (Table 2), we further analyzed these six PgCYP genes, along with the three published PgCYP genes, CYP716A47, CYP716A53v2 and CYP716A52v217–19, to have an insight into how the genes of the PgCYP gene superfamily are involved in the process. First, we functionally in silico categorized the PgCYP genes by GO term and pathway mapping to the KEGG pathway database41. Of the nine PgCYP genes, eight were assigned to one or more GO terms and categorized into all three primary functional categories, biological process (BP), molecular function (MF) and cellular component (CC) (Fig. 1A). All of them had categorized functions in Binding and Catalytic Activity (Fig. 1B; Supplementary Table S7), suggesting their biochemical function. Nevertheless, none of these six PgCYP genes and the three published ginsenoside biosynthesis PgCYP genes was mapped to any of the metabolic pathways related to ginsenoside biosynthesis documented in the KEGG pathway database. This result suggested that additional research remains to further develop the pathway(s) responsible for ginsenoside biosynthesis.
Figure 1.
GO functional categorization of the six ginsenoside biosynthesis candidate PgCYP genes and the three published ginsenosides biosynthesis PgCYP genes (CYP716A47, CYP716A53v2 and CYP716A52v2). (A) Venn diagram of eight of the PgCYP genes among the three primary functional categories: biological process (BP), molecular function (MF) and cellular component (CC). One of the PgCYP genes was not assigned to a GO term. (B) The eight PgCYP genes were categorized into seven functional subcategories (Level 2), including 2 MF subcategories, 3 CC subcategories and 2 BP subcategories.
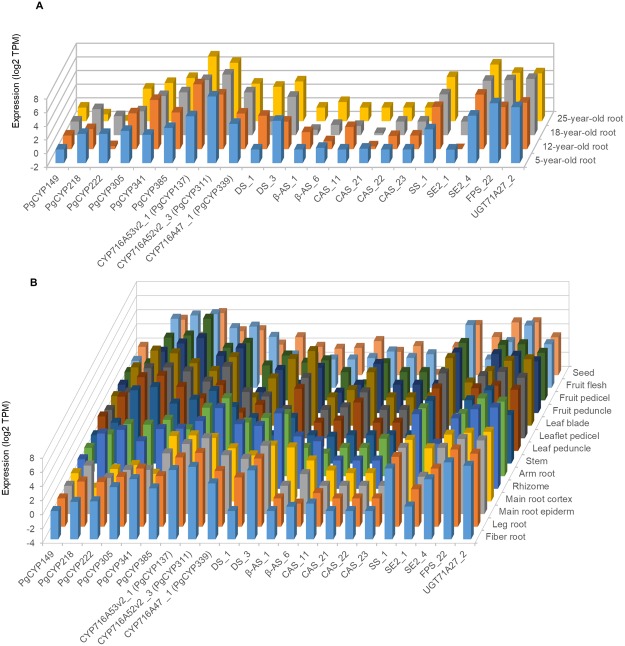
Next, we examined the expression activities of the six PgCYP genes identified in this study and those 10 published ginsenoside biosynthesis genes, including three PgCYP genes and seven genes previously cloned from other gene families, at different developmental stages and in different tissues. In the roots of differently-aged plants, from 5 to 25 years old, all 16 of the genes but PgCYP149 expressed at a developmental stage, with a variation from < 1.0 TPM to 220 TPM (Fig. 2A; Supplementary Table S8). In the different tissues of a four-year-old plant, all of the 16 analyzed genes expressed in four or more tissues, varying from < 1.0 TPM to 180 TPM, while for a same gene, its expression also varied dramatically across tissues (Fig. 2B; Supplementary Table S8).
Figure 2.
Temporal-spatial expressions of the six ginsenoside biosynthesis candidate PgCYP genes and the previously cloned ginsenosides biosynthesis genes. CYP716A53v2_1, CYP716A52v2_3, CYP716A47_1, DS_1, DS_3, β-AS_1, β-AS_6, CAS_11, CAS_21, CAS_22, CAS_23 SS_1, SE2_1, SE2_4, FPS_22 and UGT71A27_2, are previously cloned ginsenosides biosynthesis genes, while the six ginsenoside biosynthesis candidate PgCYP genes were identified in this study. The suffix of each gene, such as “_1” and “_3” to DS, indicates the transcript of the gene. (A) Expressions of the genes in the roots of differently-aged plants. (B) Expressions of the genes in different tissues of a four-year-old plant.
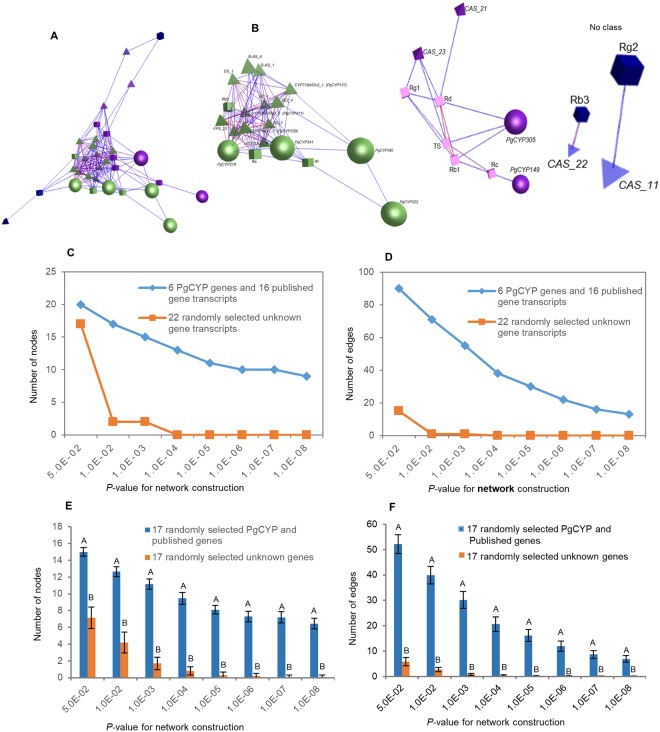
Third, we studied whether these genes, including those PgCYP genes identified in this study and those previously published, were related in functionality in ginsenoside biosynthesis. This study would also provide an additional line of evidence on whether these PgCYP genes are involved in ginsenoside biosynthesis. This is because the genes involved in a biological process or controlling a phenotype significantly tend to form a single co-expression network (MPZ, Y.-H. Liu and H.-B Zhang, personnel communication). Therefore, we constructed the co-expression network of these genes in the four-year-old roots of the 42 cultivars with the nine mono- and total-ginsenoside contents (Fig. 3A). The network consisted of all the six newly identified ginsenoside biosynthesis candidate PgCYP genes, all 10 published ginsenoside biosynthesis genes (16 transcripts) and all nine mono-ginsenosides and total ginsenoside, 184 co-expression edges and 2 clusters (Fig. 3B). The ginsenoside biosynthesis candidate PgCYP genes identified in this study closely co-expressed with the published ginsenoside biosynthesis genes and the mono- and total-ginsenoside contents in both the entire network and its individual clusters. The network formation of these genes was far more tended than those randomly-selected unknown ginseng genes (Fig. 3C–F), thus further verifying that these PgCYP genes were involved in ginsenoside biosynthesis and suggesting that the PgCYP genes are correlated in functionality in the process.
Figure 3.
Network analysis of the six ginsenoside biosynthesis candidate PgCYP genes and all 10 published ginsenoside biosynthesis genes. The network was constructed based on their expressions in the four-year-old roots of 42 cultivars. (A) The co-expression network constructed from the six PgCYP genes identified in this study indicated by balls, 16 transcripts of the 10 published ginsenoside biosynthesis genes indicated by diamonds, and 9 mono- and total ginsenosides indicated by cubes. The network was constructed at P ≤ 5.0E-02. (B) The clusters constituting the network. Different clusters are indicated by different colors. (C) Tendency that these ginsenoside biosynthesis related genes form a network, with the randomly-selected ginseng unknown genes as controls: variation in number of nodes. (D) Tendency that these ginsenoside biosynthesis related genes form a network, with the randomly-selected ginseng unknown genes as controls: variation in number of edges. (E) Statistics of variation in number of nodes in the network. (F) Statistics of variation in number of edges in the network. Different capital letters, significant at P ≤ 0.01. Error bar, standard deviation for 20 replications.
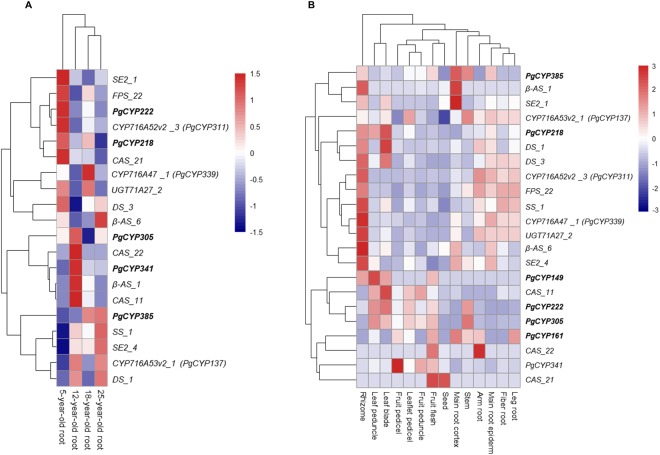
Finally, we investigated whether these genes were commonly regulated, with an attempt to identify expression signatures that are associated with a particular developmental stage or tissue through expression heatmap analysis. The result showed that common regulations existed at different developmental stages of roots for some of the genes (Fig. 4A), but were not identified in different tissues of a four-year-old plant (Fig. 4B). For instance, it appeared that β-AS_1 and CAS_11 were commonly regulated in the roots of ginseng aged from 5 years old through 25 years old (Fig. 4A). However, the expression patterns of these genes were well distinguished among the roots of differently aged plants (Fig. 4A) and the different tissues of a four-year-old plant (Fig. 4B). The unique expression patterns of these genes in differently-aged roots and different tissues, therefore, provide useful signatures for ginseng research and applications in ginsenoside biosynthesis and production.
Figure 4.
Expression heatmap of the six ginsenoside biosynthesis candidate PgCYP genes in different aged roots and tissues. The six ginsenoside biosynthesis candidate PgCYP genes newly identified in this study are bolded. All published genes involved in ginsenosides biosynthesis, CYP, AS, CAS, SE, SS, UGT, FPS and DS, were used as controls. (A) Heatmap of the genes expressed in the roots of differently-aged plants. One of the six ginsenoside biosynthesis candidate PgCYP genes did not express in the roots. (B) Heatmap of the genes expressed in the tissues of a four-year-old plant.
Discussion
The CYP genes encoding cytochrome P450 play important roles in numerous biological processes in plants and other organisms. Han et al.17–19 identified three CYP genes, CYP716A53v2, CYP716A52v2 and CYP716A47, from P. ginseng and showed that they are involved in ginsenoside biosynthesis. This study has identified 100 PgCYP genes including these three published CYP genes whose transcript expressions are significantly correlated with the variations of one or more nine mono- and/or total-ginsenoside contents in 42 ginseng cultivars. Given that the functions of all three published ginsenoside biosynthesis CYP genes, CYP716A53v2, CYP716A52v2 and CYP716A4717–19, were determined by gene expression analysis with RNAi followed by association of the repressed gene expression with the ginsenoside contents, the gene transcript expression – ginsenoside content correlation result provides a strong line of evidence on the contributions of these 97 new PgCYP genes identified in this study to ginsenoside biosynthesis. Furthermore, of these 97 PgCYP genes newly identified in this study, six were also identified to contain SNPs and/or InDels that significantly increased or decreased the content(s) of one or more mono- and/or total-ginsenosides in the four-year-old roots of ginseng cultivars (P ≤ 1.0E-04). Moreover, the six PgCYP genes newly identified in this study formed a single strong co-expression network with all the published ginsenoside biosynthesis genes. If the gene expression-ginsenoside content correlation, gene mutation-ginsenoside content variation association and newly identified PgCYP gene-published ginsenoside biosynthesis genes co-expression network are taken into account as three of the criteria for the genes involved in ginsenoside biosynthesis, this study has identified at least 6 new PgCYP genes involved in ginsenoside biosynthesis. Therefore, these six PgCYP genes have provided gene resources important for ultimate identification of the PgCYP gene involved in ginsenoside biosynthesis and the PgCYP genic SNPs/InDels have provided useful DNA markers for enhanced ginseng research and breeding.
Systems analysis of these six newly identified ginsenoside biosynthesis candidate PgCYP genes, along with the ten ginsenoside biosynthesis genes previously cloned from the PgCYP and other gene families, and the ginsenoside contents, provides a first genome-wide insight into how the PgCYP gene superfamily is involved in ginsenoside biosynthesis. First, although the six PgCYP genes all belong to the PgCYP gene superfamily and all are likely involved in the process of ginsenoside biosynthesis, they were categorized into seven GO subcategories at Level 2. Therefore, the PgCYP genes are likely involved in multiple biochemical reactions of ginsenoside biosynthesis, which is in consistence with the fact that multiple mono-ginsenosides, including the nine mono-ginsenosides investigated in this study have been found from ginseng. Second, the six PgCYP genes dramatically differentially express across tissues and developmental stages, suggesting that different members of the PgCYP genes may be responsible for ginsenoside biosynthesis individually or in group in different tissues and at different developmental stages. Third, some of the ginsenoside biosynthesis genes show different levels of common regulations across developmental stages, thus providing unique signatures for each developmental stage. Finally, the six ginsenoside biosynthesis candidate PgCYP genes and three published ginsenoside biosynthesis PgCYP genes clearly function collaboratively with each other and with those ginsenoside biosynthesis genes from other gene families in the process of ginsenoside biosynthesis, which is demonstrated by their co-expression in their network. Further, the co-expression network of these ginsenoside biosynthesis candidate genes with the published ginsenoside biosynthesis genes also suggests that the ginsenoside biosynthesis process consists of many pathways of a single network, while the different PgCYP genes are involved in different pathways of the PgCYP gene network. This may at least partly explain why this study failed to map the six ginsenoside biosynthesis candidate PgCYP genes and the three published ginsenoside biosynthesis genes17–19 to any of the KEGG metabolic pathways related to ginsenoside biosynthesis. Nevertheless, the network of the PgCYP genes provides information necessary for advanced research and applications of ginsenoside biosynthesis, such as regulation of ginsenoside biosynthesis by gene editing or RNAi and engineering of ginsenoside biosynthesis by genetic transformation.
Conclusions
Previous studies identified three PgCYP genes that are involved in the ginsenoside biosynthesis17–19. This study has identified at least six additional PgCYP genes that are highly likely involved in the ginsenoside biosynthesis. If all the PgCYP genes whose expressions were significantly correlated with the variations of nine mono- and total-ginsenoside contents in different ginseng cultivars are considered to be candidates for ginsenoside biosynthesis, the number of newly identified ginsenoside biosynthesis candidate PgCYP genes should be 97. Moreover, the six PgCYP genes form a single network with the three published PgCYP genes and seven published other genes involved in ginsenoside biosynthesis. These results, therefore, strongly suggest that these six new PgCYP genes are also involved in the ginsenoside biosynthesis and have provided a set of PgCYP genic SNP/InDel markers for advanced ginsenoside biosynthesis research and breeding in ginseng and related species.
Materials and Methods
Ginseng transcriptome databases and PgCYP genes
Three transcriptome databases of Jilin ginseng, including transcript sequences and expressions, were used for this study. The first one was developed from 14 tissues of a four-year-old ginseng plant42, the second from the roots of 5-, 12-, 18- and 25-year-old ginseng plants42 and the third from the four-year-old roots of 42 cultivars or genotypes representing the genetic variations of ginseng in its major origin and production center, Jilin, China36. These plant materials were all sampled from the major origin and production regions of ginseng in Jilin, China (Supplementary Table S3). These three transcriptome data were all sequenced using the Illumina platform Hiseq 2000 with a single flow cell, a module of 100 PE (100-nucleotide paired ends), and a sequencing depth of approximately 14 million clean reads per sample.
We previously identified 440 transcripts that were derived from 414 PgCYP genes35. These PgCYP genes were defined with numbers of 001 through 414. The different transcripts derived from a single PgCYP gene were defined by suffixing the name of the PgCYP gene with “−1”, “−2”, etc., such as PgCYP274-1 and PgCYP274-2.
Expressions of PgCYP transcripts
Since gene expression is essential for gene function and gene function determination, and different transcripts alternatively spliced from a single gene may have different biological functions34, we quantified the expression activity of each PgCYP transcript in different tissues, at different developmental stages and in different genotypes or cultivars. This was accomplished by extracting the expression profile and activity of each PgCYP transcript from the three transcriptome databases described above. The expression of each transcript was determined using the RSEM software43 bundled with the Trinity software (for detail, see ref.42). Zhang et al.44 showed that the expressions of individual transcripts quantified by shotgun RNA-seq, as measured in this study, were high reproducible between biological replicates.
Ginsenoside content assay
The contents of nine mono-ginsenosides, Rg1, Re, Rf, Rg2, Rb1, Rc, Rb2, Rb3 and Rd, were assayed by HPLC as described by Zhang et al.2. The standards for Rg1, Re, Rf, Rg2, Rb1, Rc, Rb2, Rb3 and Rd were purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). The four-year-old roots of 42 cultivars were sampled from a field trial located in Jingyu, Jilin, China and used as plant materials. The tissues used for this experiment were parts of the root tissues that were previously used to quantify the expressions of PgCYP transcripts described above36. The HPLC experiment was performed using LC-2010A HPLC equipped with LC-2010A liquid chromatography pump, LC-2010A autosampler, SHIMADZU C18 (4.6 mm × 250 mm, 5 μm) column, and CLASS-VP chromatographic workstation. We obtained the proper phenotype data for all nine mono-ginsenosides in all 42 cultivars analyzed in this study. The total ginsenoside content was the sum of the nine mono-ginsenosides.
Identification of PgCYP genes significantly correlated with ginsenoside contents
According to the central dogma of biology that the phenotype of a trait, including the ginsenoside contents in a tissue, is a result of gene expression activity, we hypothesized that the variation of ginsenoside contents in a tissue of a population must be related with the expression activity of the genes involved in ginsenoside biosynthesis. Therefore, to genome-wide identify the candidate genes that are involved in ginsenoside biosynthesis, we conducted correlation analysis between the expressions of the PgCYP gene transcripts and the variations of nine mono- and total-ginsenoside contents in the four-year-old roots of 42 cultivars using the statistical package IBM SPSS Statistics 22. The two-tailed significance levels were employed for the analysis.
Gene mutation analysis
All PgCYP genes that were significantly correlated in expression with the variations of the ginsenoside contents were subjected to analysis for genic or functional SNPs (single nucleotide polymorphisms) and InDels (nucleotide insertions and deletions) in the 42 cultivars using SAMtools45,46. The RNA-seq clean reads of each cultivar36 were used for the analysis, with the sequences of the genes expressed in 14 tissues of a four-year-old plant42 as the reference. The SNPs and InDels present only in four or more of the cultivars were used for mutation analysis. This was because the PgCYP transcripts had an average length of 1,306 bp (see below), the probability that four cultivars have a same SNP or InDel by chance, such as resulted from sequencing and/or transcript assembly errors, would be P = 3.4E-13 (1/1306*1/1306*1/1306*1/1306). This approach would allow almost all, if not all, of the SNPs or InDels that resulted from sequencing or transcript assembly errors to be excluded from the analysis. The SNPs or InDels were subjected to association analysis with the variations of the ginsenoside contents according to the single marker analysis method of QTL mapping37 and the candidate gene approach of genome-wide association study38. The SNPs and InDels that were associated with the variations of ginsenoside contents at a two-tailed significance level of P ≤ 1.0e-04 were further analyzed in open reading frame (ORF) using the ORF Finder available at NCBI.
Annotation and functional differentiation estimation of the candidate PgCYP genes involved in the ginsenoside biosynthesis
Gene ontology (GO) analysis is a widely used sequence identity-based bioinformatic tool to annotate and in silico functionally categorize genes. Therefore, to further characterize the candidate PgCYP genes for ginsenoside biosynthesis we annotated them and estimated their functional differentiation by GO analysis with the Blast2GO software47.
Co-expression network and expression heatmap analysis
The co-expression network of the ginseonside biosynthesis candidate PgCYP genes newly identified in this study, the published ginsenoside biosynthesis PgCYP genes, the published ginsenoside biosynthesis genes previously cloned from other gene families, and the nine mono- and total-ginsenoside contents, and the expression heatmaps of the PgCYP genes and the published ginsenoside biosynthesis genes were constructed as previously described42.
Electronic supplementary material
Acknowledgements
This work was supported by an award from China 863 Project (2013AA102604-3), National Natural Science Foundation of China (31401445), the Bureau of Science and Technology of Jilin Province (20170101010JC), the Development and Reform Commission of Jilin Province (2016C064).
Author Contributions
M.P.Z. designed and supervised this study, and critically revised the manuscript; Y.W. co-supervised this study and manuscript preparation; M.Z. and Y.L. performed the experiments, analyzed the data and prepared the manuscript. Y.W. assayed the ginsenoside contents. X.L., Y.H., K.W. and C.S. performed some data analysis, material sampling, plant management and assistance in research supervision. All authors approved the final manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Mingzhu Zhao and Yanping Lin contributed equally.
Contributor Information
Yi Wang, Email: wanglaoshi0606@163.com.
Meiping Zhang, Email: meiping.zhang@jlau.edu.cn.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-36349-5.
References
- 1.Choi KT. Botanical characteristics, pharmacological effects and medicinal components of Korean Panax ginseng C. A. Meyer. Acta Pharmacol. Sin. 2008;29:1109–1118. doi: 10.1111/j.1745-7254.2008.00869.x. [DOI] [PubMed] [Google Scholar]
- 2.Zhang YC, et al. Tissue-specific distribution of ginsenosides in different aged ginseng and antioxidant activity of ginseng leaf. Molecules. 2014;19:17381–17399. doi: 10.3390/molecules191117381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kenarova B, Neychev H, Hadjiivanova C, Petkov VD. Immunomodulating activity of ginsenoside Rg1 from Panax ginseng. Japa. J. Pharmacol. 1990;54:447–454. doi: 10.1254/jjp.54.447. [DOI] [PubMed] [Google Scholar]
- 4.Matsuda H, Samukawa KI, Kubo M. Anti-inflammatory activity of ginsenoside Ro1. Planta medica. 1990;56:19–23. doi: 10.1055/s-2006-960875. [DOI] [PubMed] [Google Scholar]
- 5.Mochizuki M, et al. Inhibitory effect of tumor metastasis in mice by saponins, ginsenoside-Rb2, 20 (R)-and 20 (S)-ginsenoside-Rg3, of red ginseng. Biol. Pharm. Bull. 1995;18:1197–1202. doi: 10.1248/bpb.18.1197. [DOI] [PubMed] [Google Scholar]
- 6.Sato K, et al. Inhibition of tumor angiogenesis and metastasis by a saponin of Panax ginseng, ginsenoside-Rb2. Biol. Pharm. Bull. 1994;17:635–639. doi: 10.1248/bpb.17.635. [DOI] [PubMed] [Google Scholar]
- 7.Cho WC, et al. Ginsenoside Re of Panax ginseng possesses significant antioxidant and antihyperlipidemic efficacies in streptozotocin-induced diabetic rats. Eur. J. Pharmacol. 2006;550:173–179. doi: 10.1016/j.ejphar.2006.08.056. [DOI] [PubMed] [Google Scholar]
- 8.Cheng Y, Shen LH, Zang JT. Anti-amnestic and anti-aging effects of ginsenoside Rg1 and Rb1 and its mechanism of action. Acta Pharmacol. Sin. 2005;26:143–149. doi: 10.1111/j.1745-7254.2005.00034.x. [DOI] [PubMed] [Google Scholar]
- 9.Lee MH, et al. Enhanced triterpene and phytosterol biosynthesis in Panax ginseng overexpressing squalene synthase gene. Plant Cell Physiol. 2004;45:976–984. doi: 10.1093/pcp/pch126. [DOI] [PubMed] [Google Scholar]
- 10.Kim OT, et al. Upregulation of ginsenoside and gene expression related to triterpene biosynthesis in ginseng hairy root cultures elicited by methyl jasmonate. Plant Cell Tiss. Organ Cult. 2009;98:25–33. doi: 10.1007/s11240-009-9535-9. [DOI] [Google Scholar]
- 11.Han JY, In JG, Kwon YS, Choi YE. Regulation of ginsenoside and phytosterol biosynthesis by RNA interferences of squalene epoxidase gene in Panax ginseng. Phytochemistry. 2010;71:36–46. doi: 10.1016/j.phytochem.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 12.Han JY, Kwon YS, Yang DC, Jung YR, Choi YE. Expression and RNA interference-induced silencing of the dammarenediol synthase gene in Panax ginseng. Plant Cell Physiol. 2006;47:1653–1662. doi: 10.1093/pcp/pcl032. [DOI] [PubMed] [Google Scholar]
- 13.Tansakul P, Shibuya M, Kushiro T, Ebizuka Y. Dammarenediol‐II synthase, the first dedicated enzyme for ginsenoside biosynthesis. In Panax ginseng. FEBS Lett. 2006;580:5143–5149. doi: 10.1016/j.febslet.2006.08.044. [DOI] [PubMed] [Google Scholar]
- 14.Kushiro T, Shibuya M, Ebizuka Y. β‐Amyrin synthase-Cloning of oxidosqualene cyclase that catalyzes the formation of the most popular triterpene among higher plants. Eur. J. Biochem. 1998;256:238–244. doi: 10.1046/j.1432-1327.1998.2560238.x. [DOI] [PubMed] [Google Scholar]
- 15.Kim OT, et al. Molecular characterization of ginseng farnesyl diphosphate synthase gene and its up-regulation by methyl jasmonate. Biol. Plant. 2010;54:47–53. doi: 10.1007/s10535-010-0007-1. [DOI] [Google Scholar]
- 16.Jung SC, et al. Two ginseng UDP-glycosyltransferases synthesize ginsenoside Rg3 and Rd. Plant Cell Physiol. 2014;55:2177–2188. doi: 10.1093/pcp/pcu147. [DOI] [PubMed] [Google Scholar]
- 17.Han JY, Kim HJ, Kwon YS, Choi YE. The Cyt P450 enzyme CYP716A47 catalyzes the formation of protopanaxadiol from dammarenediol-II during ginsenoside biosynthesis in Panax ginseng. Plant Cell Physiol. 2011;52:2062–2073. doi: 10.1093/pcp/pcr150. [DOI] [PubMed] [Google Scholar]
- 18.Han JY, Hwang HS, Choi SW, Kim HJ, Choi YE. Cytochrome P450 CYP716A53v2 catalyzes the formation of protopanaxatriol from protopanaxadiol during ginsenoside biosynthesis in Panax ginseng. Plant Cell Physiol. 2012;53:1535–1545. doi: 10.1093/pcp/pcs106. [DOI] [PubMed] [Google Scholar]
- 19.Han JY, Kim MJ, Ban YW, Hwang HS, Choi YE. The involvement of β-amyrin 28-oxidase (CYP716A52v2) in oleanane-type ginsenoside biosynthesis in Panax ginseng. Plant Cell Physiol. 2013;54:2034–2046. doi: 10.1093/pcp/pct141. [DOI] [PubMed] [Google Scholar]
- 20.Nelson D, Werck‐Reichhart D. A P450‐centric view of plant evolution. Plant J. 2011;66:194–211. doi: 10.1111/j.1365-313X.2011.04529.x. [DOI] [PubMed] [Google Scholar]
- 21.Matsui K, Shibutani M, Hase T, Kajiwara T. Bell pepper fruit fatty acid hydroperoxide lyase is a cytochrome P450 (CYP74B) FEBS Lett. 1996;394:21–24. doi: 10.1016/0014-5793(96)00924-6. [DOI] [PubMed] [Google Scholar]
- 22.Xiang WS, Wang XJ, Ren TR, Ju XL. Expression of a wheat cytochrome P450 monooxygenase in yeast and its inhibition by glyphosate. Pest Manage Sci. 2005;61:402–406. doi: 10.1002/ps.969. [DOI] [PubMed] [Google Scholar]
- 23.Guengerich FP. Cytochrome p450 and chemical toxicology. Chem. Res. Toxicol. 2007;21:70–83. doi: 10.1021/tx700079z. [DOI] [PubMed] [Google Scholar]
- 24.Pinot F, Beisson F. Cytochrome P450 metabolizing fatty acids in plants: characterization and physiological roles. FEBS J. 2011;278:195–205. doi: 10.1111/j.1742-4658.2010.07948.x. [DOI] [PubMed] [Google Scholar]
- 25.McLean KJ, Hans M, Munro AW. Cholesterol, an essential molecule: diverse roles involving cytochrome P450 enzymes. Biochem. Soc. Trans. 2012;40:587–593. doi: 10.1042/BST20120077. [DOI] [PubMed] [Google Scholar]
- 26.Villa-Ruano N, et al. Cytochrome P450 from plants: platforms for valuable phytopharmaceuticals. Tropical. J. Pharm. Res. 2015;14:731–742. doi: 10.4314/tjpr.v14i4.24. [DOI] [Google Scholar]
- 27.Ro DK, et al. Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature. 2006;440:940–943. doi: 10.1038/nature04640. [DOI] [PubMed] [Google Scholar]
- 28.Paddon CJ, et al. High-level semi-synthetic production of the potent antimalarial artemisinin. Nature. 2013;496:528–532. doi: 10.1038/nature12051. [DOI] [PubMed] [Google Scholar]
- 29.Fraatz MA, Berger RG, Zorn H. Nootkatone-a biotechnological challenge. Appl. Microbiol. Biot. 2009;83:35–41. doi: 10.1007/s00253-009-1968-x. [DOI] [PubMed] [Google Scholar]
- 30.Shibuya M, et al. Identification of β‐amyrin and sophoradiol 24‐hydroxylase by expressed sequence tag mining and functional expression assay. FEBS J. 2006;273:948–959. doi: 10.1111/j.1742-4658.2006.05120.x. [DOI] [PubMed] [Google Scholar]
- 31.Carelli M, et al. Medicago truncatula CYP716A12 is a multifunctional oxidase involved in the biosynthesis of hemolytic saponins. Plant Cell. 2011;23:3070–3081. doi: 10.1105/tpc.111.087312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yan Y, Kohli A, Koffas MA. Biosynthesis of natural flavanones in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2005;71:5610–5613. doi: 10.1128/AEM.71.9.5610-5613.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li R, et al. Functional genomic analysis of alkaloid biosynthesis in Hyoscyamus niger reveals a cytochrome P450 involved in littorine rearrangement. Chem. Biol. 2006;13:513–520. doi: 10.1016/j.chembiol.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 34.Syed NH, Kalyna M, Marquez Y, Barta A, Brown JW. Alternative splicing in plants–coming of age. Trends Plant Sci. 2012;17:616–623. doi: 10.1016/j.tplants.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang, Y. et al. Structural variation, functional differentiation, and activity correlation of the cytochrome P450 gene superfamily revealed in ginseng. Plant Genome, 10.3835/plantgenome2017.11.0106 (2018). [DOI] [PubMed]
- 36.Yin R, et al. Functional differentiation and spatial-temporal co-expression networks of the NBS-encoding gene family in Jilin ginseng, Panax ginseng C.A. Meyer. PloS one. 2017;12:e0181596. doi: 10.1371/journal.pone.0181596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu, B. H. Statistical genomics: Linkage, Mapping, and QTL Analysis: CRC press (1997).
- 38.Zhu C, Gore M, Buckler ES, Yu J. Status and prospects of association mapping in plants. Plant Genome. 2008;1:5–20. doi: 10.3835/plantgenome2008.02.0089. [DOI] [Google Scholar]
- 39.Graur, D. & Li, W. Rates and patterns of nucleotide substitution. Fundamentals of Molecular Evolution 99–164 (2000).
- 40.Chamary JV, Parmley JL, Hurst LD. Hearing silence: non-neutral evolution at synonymous sites in mammals. Nat. Rev. Genet. 2006;7:98–108. doi: 10.1038/nrg1770. [DOI] [PubMed] [Google Scholar]
- 41.Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang K, et al. The spatial and temporal transcriptomic landscapes of ginseng, Panax ginseng C.A. Meyer. Scientific Rep. 2015;5:18283. doi: 10.1038/srep18283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li B, Dewey CN. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics. 2011;12:323. doi: 10.1186/1471-2105-12-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang, M. P. et al. Quantification of gene expression while taking into account RNA alternative splicing. Genomics, 10.1016/j.ygeno.2018.10.009 (2018). [DOI] [PubMed]
- 45.Li H, et al. The sequence alignment/map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li H. A statistical framework for SNP calling, mutation discovery, association mapping and population genetical parameter estimation from sequencing data. Bioinformatics. 2011;27:2987–2993. doi: 10.1093/bioinformatics/btr509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ashburner M, et al. Gene Ontology: tool for the unification of biology. Nat. Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.