Figure 2.
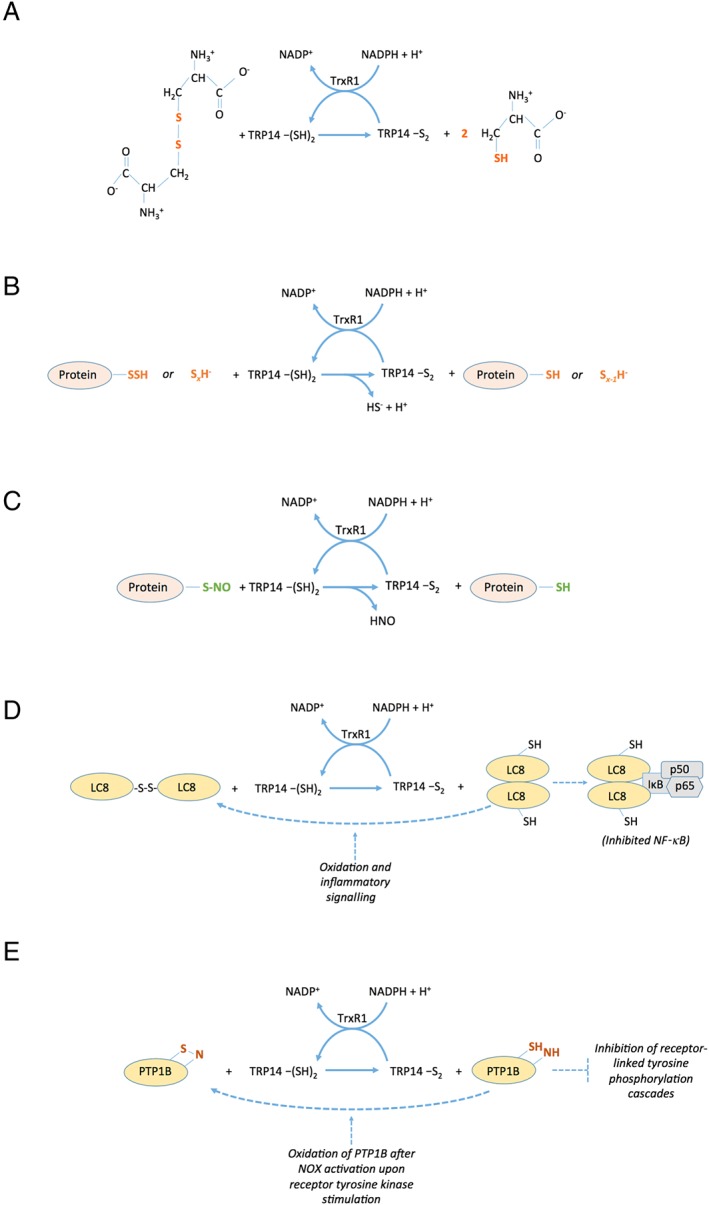
Schematic summary of selected enzymatic reactions catalyzed by TRP14. As is further discussed in the text, TRP14 lacks enzymatic activity with several classic Trx substrates including ribonucleotide reductase, methionine sulfoxide reductases or peroxiredoxins. However, several other enzymatic activities of TRP14, coupled to its own NADPH‐dependent reduction from an active site disulfide to a dithiol by TrxR1, were described in recent years. Some of these are summarized here, with the key features in the different substrates being reduced by TRP14 indicated in colour. The activities include (A) reduction of cystine into two molecules of cysteine, (B) reduction of persulfidated proteins or inorganic polysulfides, (C) reduction of nitrosylated Cys residues (S‐nitrosolyated moieties) in proteins, (D) reduction of a disulfide in LC8 that activates LC8 as an inhibitor of NF‐κB through interactions of reduced LC8 with IκB and (E) reduction of a presumed sulfenylamide motif in oxidized PTP1B that promotes its phosphatase activity, which inhibits receptor‐linked tyrosine phosphorylation cascades.
