Figure 4.
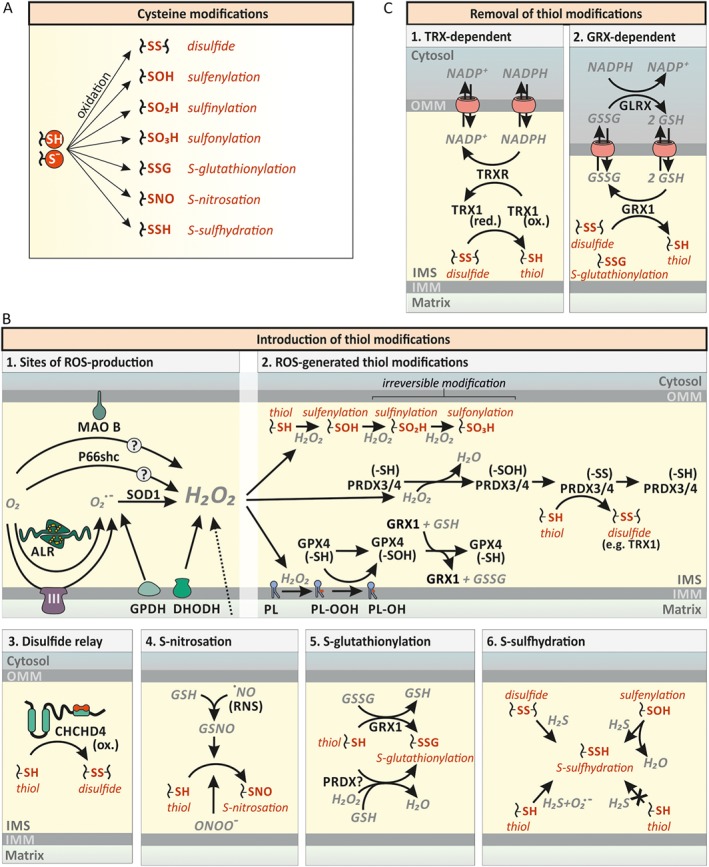
Cysteine modifications in the IMS. (A) Cysteine modifications. The reactive thiol group of cysteine residues can exist in the protonated state or as the deprotonated thiolate anion. This thiol group can be further modified as indicated. (B) Various thiol modifications are introduced into IMS proteins. (1) ROS‐generation in the IMS. Transfer of a single electron to O2 leads to formation of superoxide anion (O2°−). Superoxide generators of the IMS include complex III of the respiratory chain (CIII), the ALR, and GPDH. Superoxide is enzymically converted to H2O2 by SOD1. Dihydroorotate dehydrogenase (DHODH) can generate O2°− and H2O2. Likewise, MAO B and p66Shc have been reported to directly generate H2O2. (2) Cysteine modification by H2O2. H2O2 can result in the direct oxidation of target thiols (–SH) to sulfenic (–SOH), sulfinic (–SO2H) and sulfonic (–SO3H) acid depending on thiol reactivity and H2O2 availability. Most protein thiols exhibit low reactivity towards H2O2 and will thus be primarily metabolised by dedicated H2O2‐scavenging enzymes such as peroxiredoxins (PRDX). PRDX3/4 are located in the IMS and their oxidation by H2O2 can be reversed by thioredoxins (TRX), but might also be passed on to other proteins. H2O2 also mediates phospholipid (PL) peroxidation (PL–OOH). PL peroxidation can be removed by GSH peroxidase‐4 (GPX4). (3) The mitochondrial disulfide relay. During oxidative protein folding, reduced cysteine residues (–SH) are oxidized to disulfide bonds (–SS) by oxidized (ox.) CHCHD4. See Figure 5 for additional details. (4) S‐nitrosations of cysteine residues. Reduced GSH reacts with ·NO to S‐nitrosoglutathione (GSNO), inducing S‐nitrosations (–SNO). NO is also able to directly induce S‐nitrosations. (5) S‐glutathionylation of cysteine residues. Reduced glutaredoxin‐1 [GRX1 (–SH)] reacts with GSSG and becomes glutathionylated [GRX1 (–SSG)]. GRX1 subsequently mediates the S‐glutathionylation (–SSG). (6) S‐sulfhydration of cysteine residues. Disulfides and sulfenic acids (–SOH) can react with H2S, resulting in S‐sulfhydration (–SSH) of cysteines. Thiols on the other hand can only be sulfhydrated by H2S in the presence of superoxide anions. (C) Removal of thiol modifications. (1) Reduction of disulfide bonds by the thioredoxin system (TRX).Thioredoxin 1 (TRX1) is likely to mediate the reduction of disulfides and become oxidized itself. Thioredoxin reductase (TRXR) reduces TRX1 in an NADPH‐dependent manner. (2) Reduction of disulfide bonds and S‐glutathionylations by GRX1. GRX1 reduces disulfides and S‐glutathionylated thiols (–SSG) while oxidizing reduced GSH. GSH disulfide (GSSG) is reduced by GSH reductase (GLRX) in an NADPH‐dependent manner.
