Figure 5.
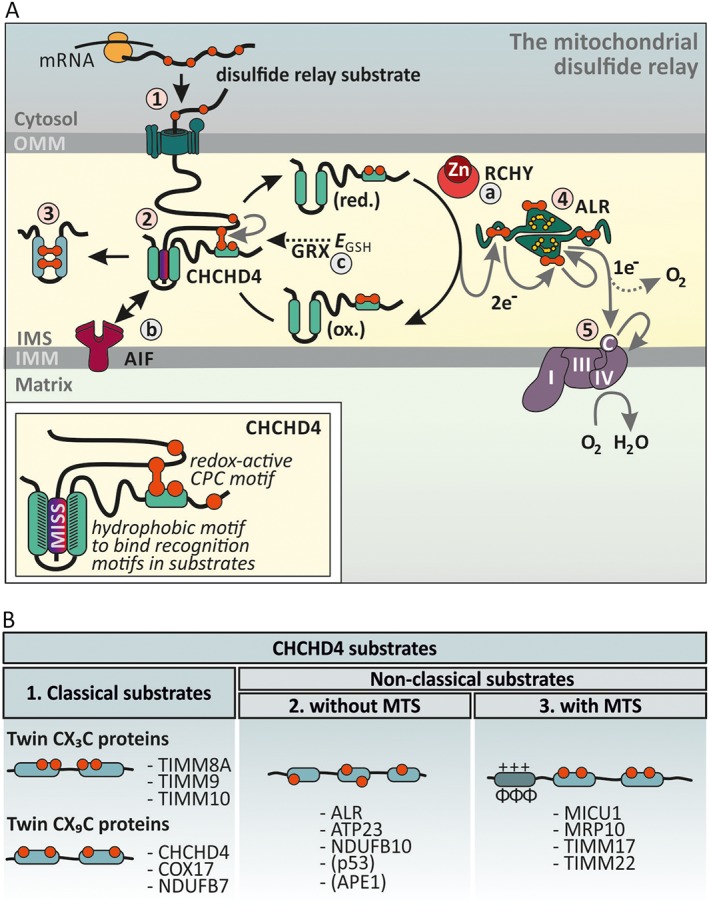
Enzyme‐catalysed disulfide formation in the IMS. (A) The mitochondrial disulfide relay. Disulfide relay substrates are synthesized on cytosolic ribosomes and are targeted to the TOM pore (step 1). During translocation, the substrates' mitochondrial IMS‐sorting signal (MISS, also ITS) binds the hydrophobic cleft of CHCHD4 and a mixed disulfide between the substrate and active site cysteines of CHCHD4 is formed (step 2). After releasing the oxidized and folded substrate (step 3), CHCHD4 is present in a reduced state. Electrons from reduced CHCHD4 are transferred to ALR (step 4). ALR shuffles electrons onto cytochrome c, which in turn transfers them to complex IV (step 5). The disulfide relay is supported by different auxiliary factors. RCHY/Hot13 complexes Zn2+ and thereby accelerates oxidation (step a). AIF facilitates CHCHD4 biogenesis and thus ensures the presence of this critical protein in the IMS (step b). The GSH redox buffer (EGSH) and glutaredoxins proofread wrongly formed or trapped disulfides. Glutaredoxins are present only in limiting amounts to allow disulfide formation (step c). (B) CHCHD4 substrates fall into three different structural classes. Proteins that lack classical mitochondrial targeting sequences and instead contain conserved cysteine residues form classes 1 and 2. The cysteines can either be ordered in twin CX3C of twin CX9C motifs (class 1) or not (class 2). Proteins from both classes acquire disulfide bonds during import, and it appears that this also is the prerequisite for mitochondrial accumulation and retention in the IMS. CHCHD4 also oxidizes substrates that rely on N‐terminal mitochondrial targeting sequences (MTS) for import (class 3).
