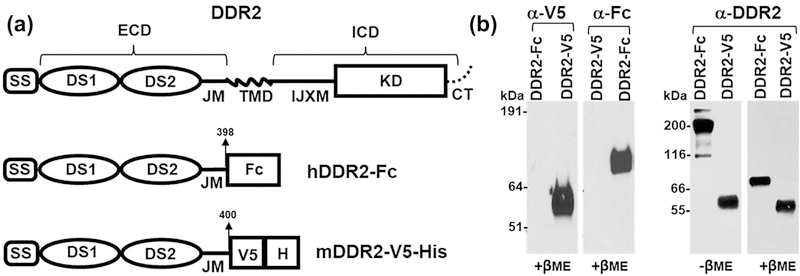
Figure 1:
(a) Schematic diagram showing the structure of full length DDR2, human DDR2-Fc and mouse DDR2-V5-His constructs. SS: signal sequence; DS: discoidin domain; ECD: extracellular domain; IJXM: intracellular juxtamembrane region; TMD: transmembrane domain; ICD: intracellular domain; KD: kinase domain (b) Purified recombinant DDR2-V5-His and DDR2-Fc proteins (20 ng/lane) were resolved by SDS-PAGE under reducing conditions (+βME), with either 4–12% (w/v) Bis-Tris Gels (left panel) or under reducing and non-reducing (-βME, 100 ng/lane)) conditions with 10% SDS-PAGE (right panel). The separated protein was detected by immunoblotting using anti-epitope or anti-DDR2 antibodies as indicated.