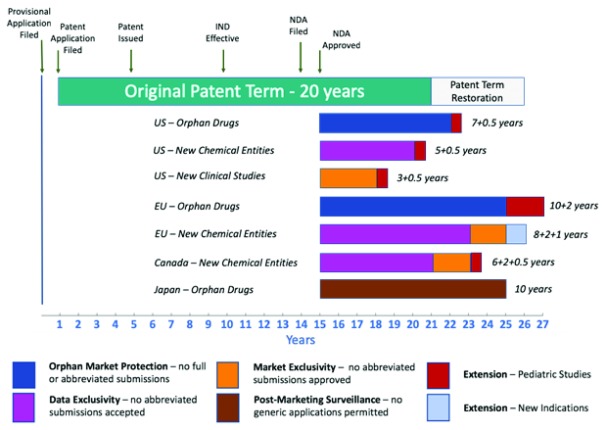
Figure 1. Comparing protection against competition for a new drug sponsor under an average effective patent term in the US with protection against competition under prospective M4K regulatory exclusivity periods in the US, EU, Canada, and Japan, using a new drug targeting DIPG as the exemplar.
The average effective composition of matter patent term for a new drug after patent restoration in the US is approximately 11–12 years ( source: Cárdenas-Navia, J. Thirty Years of Flawed Incentives: an Empirical and Economic Analysis of Hatch-Waxman Patent-Term Restoration. Berkeley Technol. Law J. 29, (2015)). In comparison, irrespective of its patent status, a new drug approved to treat DIPG could be entitled to (i) orphan drug exclusivities of 7.5 years in the US (including a 6-month paediatric extension) and 12 years in the EU (including a 2-year paediatric extension); (ii) new chemical entity exclusivities of 5.5 years in the US (including a 6-month paediatric extension), 10 years in the EU, and 8.5 years in Canada (including a 6-month paediatric extension); and (iii) a period of orphan drug post-marketing surveillance of 10 years in Japan (which acts as an equivalent bar to entry by competitors). Approval of subsequent indications for the same drug could entitle M4K to (i) 3.5 years of new clinical study exclusivity in the US (including a 6-month paediatric extension, if the new indication required further paediatric studies) and (ii) a 1-year extension of new chemical entity exclusivity in the EU (for a total of 11 years).