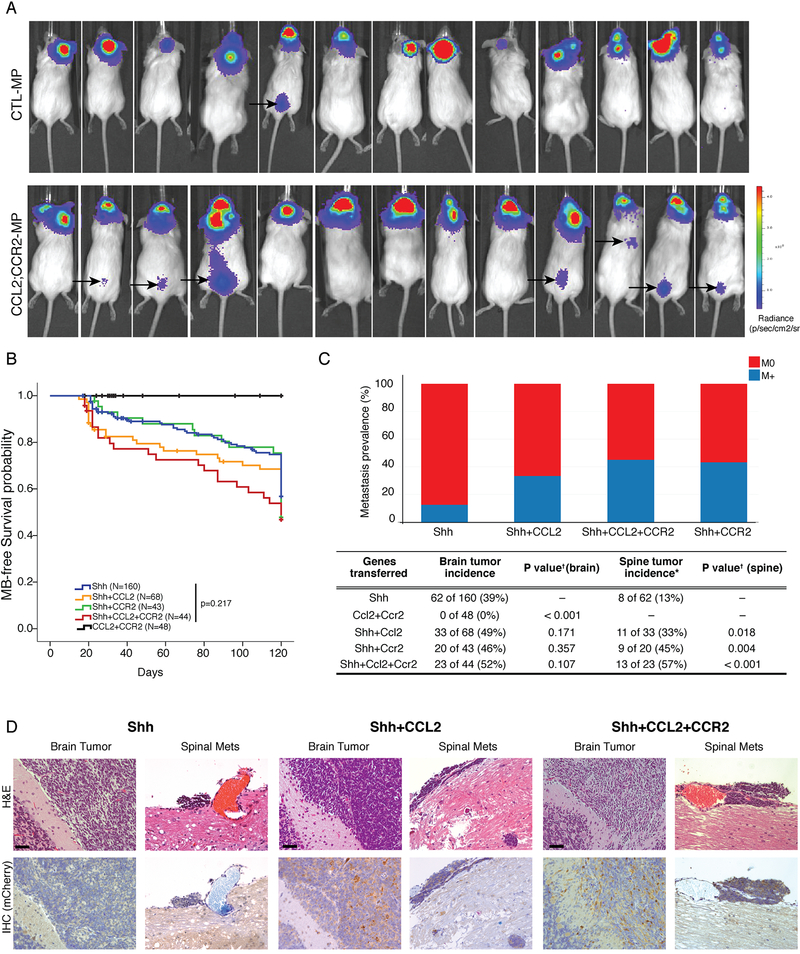
Figure 6: The CCL2/CCR2 Axis Drives Leptomeningeal Dissemination In Vivo.
NSG mice implanted with MPLuc allografts develop spinal metastasis with higher frequency when both CCL2 and CCR2 are overexpressed (1/13 vs 7/13, p=0.006 Fisher’s Exact Test with mid-P adjustment, α=0.05), the black arrows point to BLI signals from the spinal metastasis observable 15 days after implantations of the tumor cells (See also Figure S6). b) Nestin-TVA transgenic mice were injected with viruses to transfer either Shh alone, Shh+CCL2, Shh+CCR2, Shh+CCL2+CCR2, or CCL2+CCR2 alone. Mice injected with CCL2+CCR2 did not develop medulloblastoma. All other genotypes were observed to have a similar rate of medulloblastoma free survival (Log-Rank test p=0.217). c) While Shh virus alone leads to localized medulloblastoma in vivo, the addition of CCL2 and/or CCR drives the development of leptomeningeal metastases. Expression of CCL2, or CCR2 has no significant impact on the incidence of primary tumor formation (Chi-square test p=0.107), but does have a significant and dramatic effect on the incidence of leptomeningeal metastases (Chi-square test p=0.018). d) Primary and metastatic medulloblastoma sections from Nestin-TVA mice infected with Shh and mCherry-CCL2 viruses demonstrates that CCL2 expression is subclonal in the primary tumor, but is highly clonally selected and ubiquitous in the metastases. e) Primary and metastatic medulloblastoma sections from Nestin-TVA mice infected with Shh, Shh+CCL2, and Shh+ CCL2+CCR2 viruses demonstrates that CCL2 expression - revealed by IHC for CLL2 mcherry tag - is subclonal in the primary tumor, but is clonally selected in the meningeal metastasis. Scale bars 50μm.