Abstract
Cu/TEMPO catalyst systems promote efficient aerobic oxidation of sterically unhindered primary alcohols and electronically activated substrates, but they show reduced reactivity with aliphatic and secondary alcohols. Here, we report a catalyst system, consisting of (MeObpy)CuI(OTf) and ABNO (MeObpy =4,4′-dimethoxy-2,2′-bipyridine; ABNO = 9-azabicyclo[3.3.1]nonane N-oxyl), that mediates aerobic oxidation of all classes of alcohols, including primary and secondary allylic, benzylic, and aliphatic alcohols with nearly equal efficiency. The catalyst exhibits broad functional group compatibility, and most reactions are complete within 1 h at room temperature using ambient air as the source of oxidant.
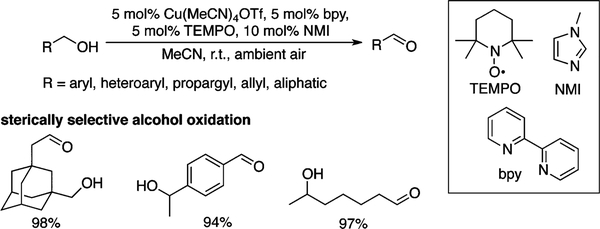
The oxidation of alcohols to carbonyl compounds is one of the most common classes of oxidation reactions in organic chemistry. Widespread interest has been directed toward the development of catalytic aerobic oxidation methods,1 but few of these reactions approach the synthetic utility and/or scope of traditional stoichiometric oxidants,2 such as chromium oxides, Dess-Martin periodinane, or activated DMSO methods. Copper/TEMPO catalyst systems have begun to address this limitation (TEMPO = 2,2,6,6-tetramethyl-1-piperidinyloxyl).3 We recently reported a (bpy)CuI/TEMPO/NMI catalyst system (bpy = 2,2′-bipyridine; NMI = N-methylimidazole) that is highly effective for aerobic oxidation of primary alcohols to aldehydes (Scheme 1).3h This method tolerates diverse functional groups and exhibits excellent steric discrimination between unprotected primary and secondary alcohols as well as primary alcohols in different steric environments (cf. Scheme 1). This steric sensitivity addresses important chemoselectivity challenges, but it also limits the scope of the method to primary alcohols. Here, we report a complementary copper/ABNO catalyst system (ABNO = 9-azabicyclo[3.3.1]nonane N-oxyl) that displays excellent reactivity and functional group compatibility with all classes of alcohols.
Scheme 1.
Selective Aerobic Oxidation of Primary Alcohols with a (bpy)CuI/TEMPO/NMI Catalyst System
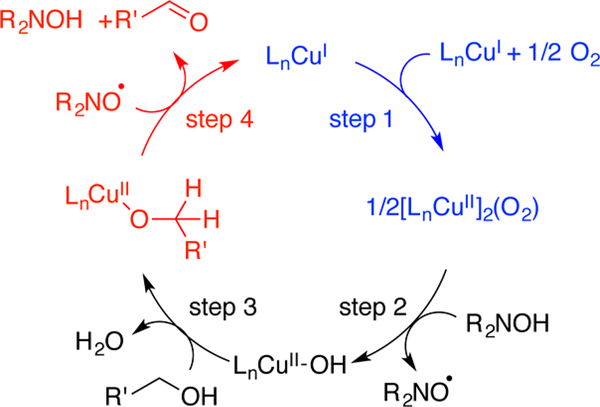
The (bpy)CuI/TEMPO/NMI catalyst system is significantly more reactive with allylic, benzylic, and other activated alcohols than with aliphatic substrates. Activated alcohols often reach completion within 2–3 h at room temperature, while aliphatic alcohols can require up to 24 h and/or elevated reaction temperatures. Recent mechanistic studies provide insights into the origin of these results.4 Aliphatic alcohols undergo turnover-limiting C–H cleavage via a bimolecular reaction of TEMPO with a CuII-alkoxide intermediate (step 4, Scheme 2), as revealed by kinetic studies and isotope effects. In contrast, the turnover-limiting step with activated alcohols is oxidation of CuI by O2 (step 1, Scheme 2). This change arises from the substantially weaker α-C–H bonds of activated alcohols, leading to much more rapid C–H cleavage.
Scheme 2.
Simplified Catalytic Cycle for CuI/TEMPO-Catalyzed Aerobic Alcohol Oxidation
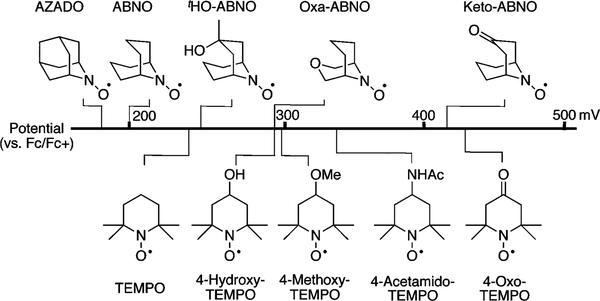
We speculated that enhanced catalytic activity could be achieved with aliphatic and/or secondary alcohols by replacing TEMPO with a nitroxyl cocatalyst having different electronic or steric properties. Diverse nitroxyl derivatives with different redox properties have been reported in the literature (Figure 1),5−8 and various bicyclic nitroxyls (Figure 1, top row) have a smaller steric profile than TEMPO analogs. Our studies commenced by testing various nitroxyl derivatives with (bpy)CuI/NMI for aerobic oxidation of cyclohexanemethanol.
Figure 1.
Diverse nitroxyl derivatives and their one-electron oxoammonium/nitroxyl redox potential (vs Fc/Fc+ in MeCN).
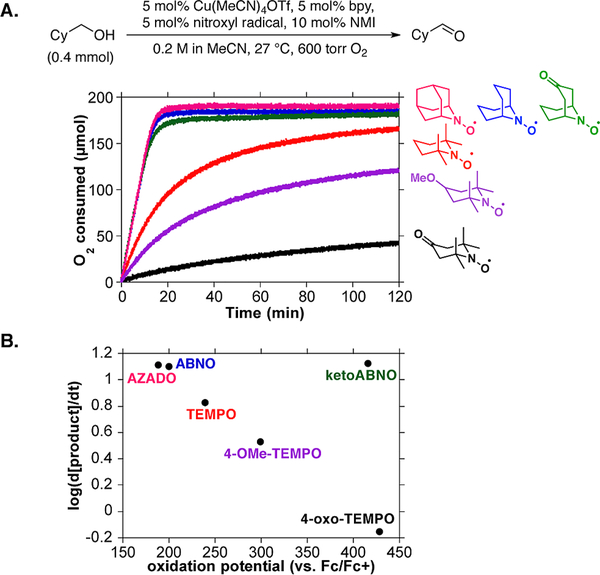
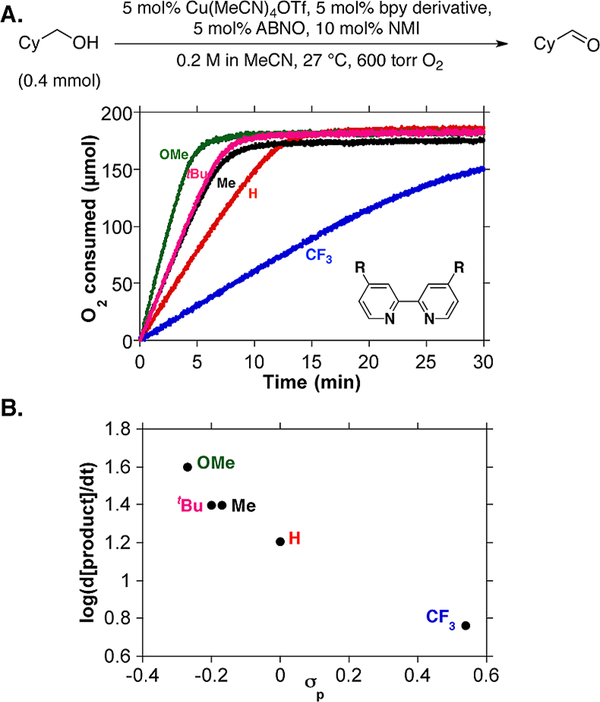
The reactions were analyzed by monitoring O2 consumption with a gas-uptake apparatus (Figure 2A). Testing of TEMPO derivatives revealed decreases in rate with more-oxidizing derivatives (Figure 2B). In contrast, no dependence on redox potential was observed with the less sterically encumbered bicyclic nitroxyl derivatives AZADO, ABNO, and ketoABNO, and the rates were uniformly higher than those with TEMPO derivatives (Figure 2A and 2B). The mechanistic origin of these results is the focus of ongoing investigation, but several important preliminary observations can be made. The linear reaction time courses with bicylic nitroxyls (cf. Figure 2A) imply a zero-order dependence of the rate on [alcohol]. The lack of dependence of the rate on the nitroxyl redox potential suggests the nitroxyl is not involved in the turnover-limiting step. Consistent with this conclusion, the ABNO loading could be reduced to 1 mol % without affecting the rate. These kinetic features resemble Cu/TEMPO-catalyzed oxidation of activated alcohols (see above) and differ from Cu/TEMPO-catalyzed oxidation of aliphatic alcohols, which shows a dependence on both [TEMPO] and [alcohol]. These observations suggest that less sterically hindered nitroxyls significantly enhance the rate of C–H cleavage and lead to a new turnover-limiting step.
Figure 2.
Comparison of six different nitroxyl radicals in the oxidation of cyclohexanemethanol.
In order to explore the synthetic consequences of the results described above, we optimized a catalyst system with ABNO as the cocatalyst. Comparison of different Cu sources confirmed that CuI salts, particularly those with noncoordinating anions, were most effective (Table S1). Numerous mono- and bidentate nitrogen ligands were evaluated in combination with CuIOTf/ABNO (Tables S2 and S3), but the best results were still obtained with a bpy derivative in combination with NMI. Nearly identical results were obtained when NMI was replaced with 4-dimethylaminopyridine (DMAP). Electron-rich bpy derivatives enhanced the reaction rate (Figure 3), and the best results were observed with 4,4′-dimethoxy-2,2′-bipyridine (MeObpy).9
Figure 3.
Comparison of five different bpy derivatives in the oxidation of cyclohexanemethanol.
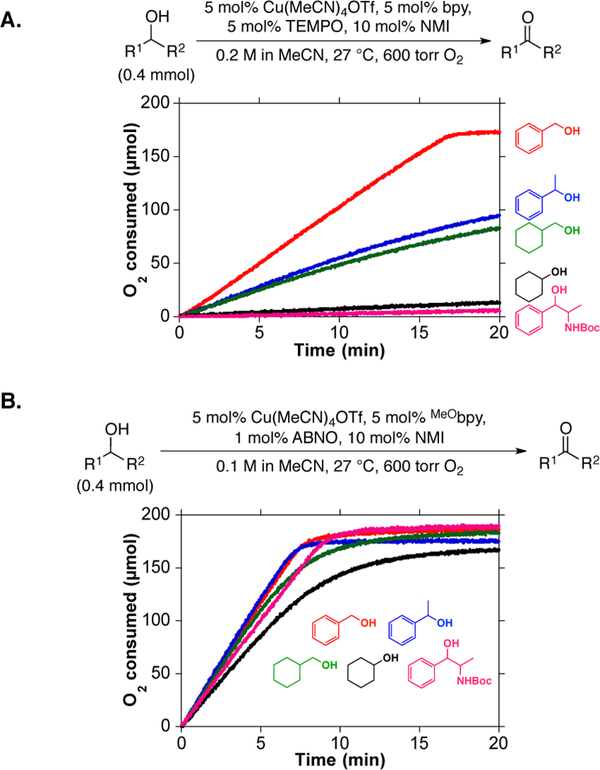
This new catalyst system was tested with a representative set of primary and secondary benzylic and aliphatic alcohols (Figure 4). Direct comparison of reactions with CuI/TEMPO and CuI/ABNO highlights significant differences in the substrate scope of these two catalyst systems. CuI/TEMPO shows strong sensitivity to substrate electronic and steric properties (Figure 4A). Primary aliphatic and all secondary alcohols proceed with much slower reaction rates than benzyl alcohol; cyclohexanol and N-Boc-norephedrine are essentially unreactive under these conditions. In contrast, CuI/ABNO shows excellent reactivity with all substrates (Figure 4B) and promotes complete conversion within approximately 10 min at room temperature in each case. These observations show that replacement of TEMPO with the less sterically encumbered ABNO alleviates the steric and electronic constraints of the Cu/TEMPO catalyst system.
Figure 4.
Rate comparison of five different alcohols with CuI/TEMPO and CuI/ABNO alcohol oxidation systems.
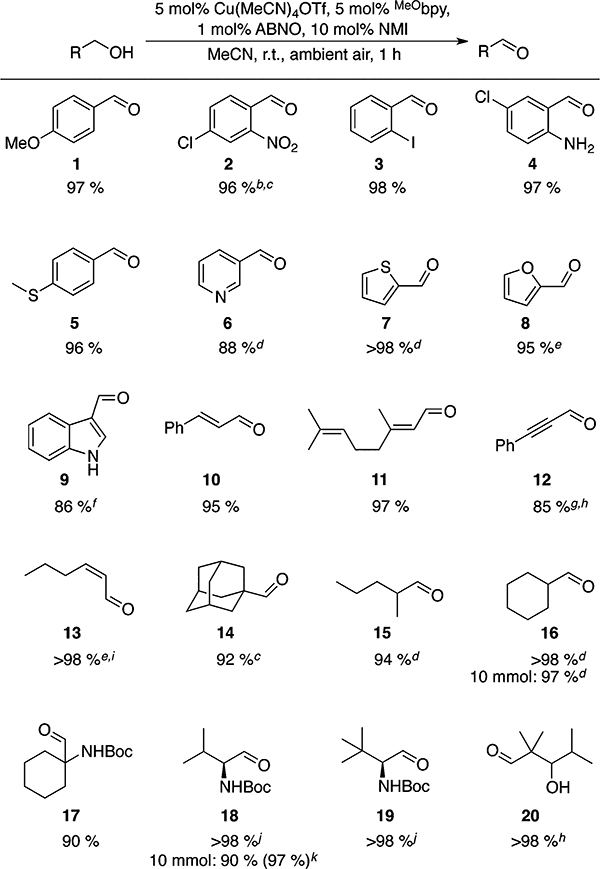
Excellent product yields are obtained in the oxidation of a wide range of primary alcohols bearing diverse functional groups, including ethers, thioethers, heterocycles, amines, alkenes, and alkynes (Table 1). In nearly all cases, the reactions are complete within 1 h at room temperature under ambient air (i.e., in a reaction vessel open to the air). Consistent with the data in Figure 4, no significant differences are observed between benzylic, allylic, and aliphatic substrates. Somewhat slower rates are observed with 3-hydroxymethylindole, which affords aldehyde 9 in 3 h, and the highly electron-deficient 4-chloro-2-nitrobenzyl alcohol, which forms 2 in near-quantitative yield upon heating at 50 °C for 2 h.10 Some limitations of the method resemble those observed with the CuI/TEMPO catalyst.3h For example, reaction inhibition and/or poor yields are observed with phenols, primary homobenzylic alcohols, and substrates bearing terminal alkynes (data not shown). Nevertheless, the reaction tolerates many oxidatively sensitive functional groups, such as internal alkenes and alkynes as well as thioethers. Oxidation of Boc-protected β-aminoalcohols proceeds without epimerization of the stereocenter (cf. 18 and 19), and a (Z)-allylic alcohol undergoes oxidation with complete retention of the cis double bond configuration (13).11 These observations draw attention to the mildness of the catalytic reaction conditions.
Table 1.
Scope of (MeObpy)CuI/ABNO-Catalyzed Aerobic Primary Alcohol Oxidationa
Yields given are for isolated material. All reactions were performed with 1 mmol of alcohol in 10 mL of MeCN for 1 h in an open reaction vessel, unless otherwise noted.
Reaction performed at 50 °C for 2.2 h.10
1.5% RSM by 1H NMR spectroscopy.
Isolated as the 2,4-dinitrophenylhydrazone due to product volatility.
Isolated as a solution in pentane.
Reaction run for 3 h. 4 % RSM by 1H NMR spectroscopy.
2.5% RSM by 1H NMR spectroscopy.
O2 balloon used.10
>19:1 Z/E.
No loss in enantiopurity was observed (see Supporting Information (SI) for details).
1H NMR yield.
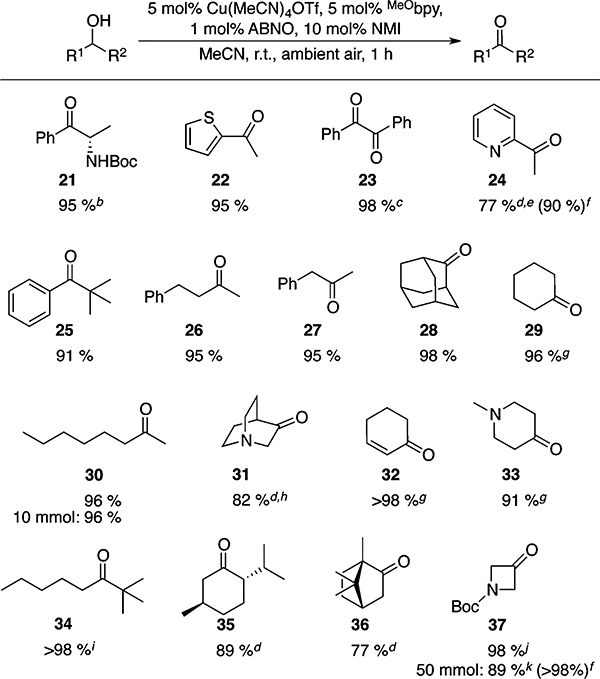
Further testing of the CuI/ABNO protocol revealed a similarly broad scope and catalytic efficiency with diverse secondary alcohols, including allylic, benzylic, and aliphatic substrates (Table 2). The reactions display functional-group compatibility similar to that observed with primary alcohols, including tolerance of amines as well as sulfur and nitrogen heterocycles. Once again, most of the reactions afford the product within 1 h at room temperature under ambient air (Table 2). Various sterically encumbered substrates undergo oxidation in excellent yields (cf. 25, 34–36), a feature that likely reflects the steric accessibility of ABNO. Whereas primary homobenzylic alcohols lead to overoxidation with CuI/TEMPO and CuI/ABNO conditions, the secondary homobenzylic alcohol proceeds in excellent yield to ketone 27 without side products.
Table 2.
Scope of (MeObpy)CuI/ABNO-Catalyzed Aerobic Secondary Alcohol Oxidationa
Yields given are for isolated material. All reactions were performed on a 1 mmol scale in 10 mL of MeCN for 1 h in an open reaction vessel, unless otherwise noted.
>99% ee. 0.75 h reaction time.
Benzoin used as starting material.
Reaction performed at 70 °C with O2 balloon.10
2 h reaction time; 2 mol % catalyst added after 1.5 h (see SI for details).
1H NMR yield.
Isolated as the 2,4-dinitrophenylhydrazone due to product volatility.
Isolated as the HCl salt.
Reaction performed at 60 °C.10
0.5 reaction time.
0.2 M substrate concentration.
This CuI/ABNO protocol is readily amenable to larger-scale application. The oxidation of cyclohexanemethanol, 2-octanol, and N-Boc-valinol were performed on a 10 mmol (1–2 g) scale and afforded products 16, 18, and 30 in ≥90% yield. In each case, the reaction was carried out in an open reaction flask at room temperature and were complete within 1 h. The N-Boc-azetidinone product 37 was prepared on a 50 mmol scale (approximately 9 g) in a similar manner, albeit with a somewhat longer reaction time (3 h). Reactions with a 1 mol % MeObpy/ Cu loading suggest that the catalyst deactivates before reaching full conversion of the substrate (cf. Table S1). The origin of this deactivation and efforts to overcome it are the focus of ongoing work.
In summary, we have identified a highly effective, broad-scope catalyst system for aerobic alcohol oxidation. Numerous practical features associated with this method should facilitate its use in synthetic chemistry, including fast reaction rates, mild reaction conditions, compatibility with air as the source of oxidant, and use of a common organic solvent (acetonitrile). The CuI/ABNO catalyst complements the recently developed CuI/TEMPO catalyst system. Whereas CuI/TEMPO shows high chemoselectivity for primary alcohols, CuI/ABNO is equally effective with all classes of alcohol substrates. Together, these catalyst systems provide compelling aerobic alternatives to traditional alcohol oxidation methods.
Supplementary Material
ACKNOWLEDGMENTS
Financial support for this work was provided by the DOE (DEFG02-05ER15690), ACS GCI Pharmaceutical Roundtable, a consortium of pharmaceutical companies (Eli Lilly, Pfizer, and Merck), and the NSF (predoctoral fellowship to J.E.S.). NMR spectroscopy facilities were partially supported by the NSF (CHE-9208463) and NIH (S10 RR08389).
Footnotes
Notes
The authors declare no competing financial interest.
Supporting Information
Full catalyst screening data, experimental procedures, product characterization data for all products. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- (1).(a) For reviews, see: Sheldon RA; Arends IWCE; ten Brink G-J; Dijksman A Acc. Chem. Res 2002, 35, 774–781. [DOI] [PubMed] [Google Scholar]; (b) Mallat T; Baiker A Chem. Rev. 2004, 104, 3037–3058. [DOI] [PubMed] [Google Scholar]; (c) Zhan B-Z; Thompson A Tetrahedron 2004, 60, 2917–2935. [Google Scholar]; (d) Schultz MJ; Sigman MS Tetrahedron 2006, 62, 8227–8241. [Google Scholar]; (e) Matsumoto T; Ueno M; Wang N; Kobayashi S Chem.— Asian J 2008, 3, 196–214. [DOI] [PubMed] [Google Scholar]; (f) Parmeggiani C; Cardona F Green Chem. 2012, 14, 547–564. [Google Scholar]
- (2).(a) Tojo G; Fernández Ḿ Oxidation of Alcohols to Aldehydes and Ketones; Springer: New York, 2010. [Google Scholar]; (b) Tojo G; Fernández M Oxidation of Primary Alcohols to Carboxylic Acids; Springer: New York, 2010. [Google Scholar]
- (3).(a) For leading references, see: Semmelhack MF; Schmid CR; Cortés DA; Chou CS, J. Am. Chem. Soc 1984, 106, 3374− 3376. [Google Scholar]; (b) Ragagnin G; Betzemeier B; Quici S; Knochel P Tetrahedron 2002, 58, 3985–3991. [Google Scholar]; (c) Gamez P; Arends IWCE; Reedijk J; Sheldon RA Chem. Commun 2003, 2414–2415. [DOI] [PubMed] [Google Scholar]; (d) Gamez P; Arends IWCE; Sheldon RA; Reedijk J Adv. Synth. Catal 2004, 346, 805–811. [Google Scholar]; (e) Jiang N; Ragauskas AJ J. Org. Chem 2006, 71, 7087–7090. [DOI] [PubMed] [Google Scholar]; (f) Kumpulainen ETT; Koskinen AMP Chem.—Eur. J 2009, 15, 10901–10911. [DOI] [PubMed] [Google Scholar]; (g) Figiel PJ; Sibaouih A; Ahmad JU; Nieger M; Räisänen MT; Leskelä M; Repo T Adv. Synth. Catal 2009, 351, 2625–2632. [Google Scholar]; (h) Hoover JM; Stahl SS J. Am. Chem. Soc 2011, 133, 16901–16910. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Könning D; Hiller W; Christmann M Org. Lett 2012, 14, 5258–5261. [DOI] [PubMed] [Google Scholar]; (j) Hoover JM; Steves JE; Stahl SS Nat. Protoc 2012, 7, 1161− 1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).(a) Hoover JM; Ryland BL; Stahl SS J. Am. Chem. Soc 2013, 135, 2357–2367. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Hoover JM; Ryland BL; Stahl SS ACS Catal, in press (DOI: 10.1021/cs400689a). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).(a) Bobbitt JM; Brückner C; Merbouh N Org. React 2009, 74, 106–132. [Google Scholar]; (b) Tebben L; Studer A Angew. Chem., Int. Ed 2011, 50, 5034–5068. [DOI] [PubMed] [Google Scholar]
- (6).(a) For early reports of bicylic nitroxyl derivatives, see: Bowman DF; Gillan T; Ingold KU J. Am. Chem. Soc 1971, 93, 6555–6561. [Google Scholar]; (b) Malatesta V; Ingold KU J. Am. Chem. Soc 1973, 95, 6404− 6407. [Google Scholar]; (c) Dupeyre R-M; Rassat A Tetrahedron Lett. 1975, 16, 1839–1840. [Google Scholar]; (d) Keana JFW Chem. Rev 1978, 78, 37–64. [Google Scholar]; (e) Nelsen SF; Kessel CR; Brien DJ >J. Am. Chem. Soc 1980, 102, 702–711. [Google Scholar]
- (7).(a) For representative applications of bicyclic nitroxyl radicals in other alcohol and amine oxidation reactions, see: Graetz B; Rychnovsky S; Leu W-H; Farmer P; Lin R Tetrahedron: Asymmetry 2005, 16, 3584–3598. [Google Scholar]; (b) Shibuya M; Tomizawa M; Suzuki I; Iwabuchi YJ Am. Chem. Soc 2006, 128, 8412–8413. [DOI] [PubMed] [Google Scholar]; (c) Demizu Y; Shiigi H; Oda T; Matsumura Y; Onomura O Tetrahedron Lett 2008, 49, 48–52. [Google Scholar]; (d) Shibuya M; Sato T; Tomizawa M; Iwabuchi Y Chem. Commun 2009, 1739–1741. [DOI] [PubMed] [Google Scholar]; (e) Tomizawa M; Shibuya M; Iwabuchi Y Org. Lett 2009, 11, 1829–1831. [DOI] [PubMed] [Google Scholar]; (f) Shibuya M; Osada Y; Sasano Y; Tomizawa M; Iwabuchi YJ Am. Chem. Soc 2011, 133, 6497–6500. [DOI] [PubMed] [Google Scholar]; (g) Hayashi M; Shibuya M; Iwabuchi YJ Org. Chem 2012, 77, 3005–3009. [DOI] [PubMed] [Google Scholar]; (h) Shibuya M; Doi R; Shibuta T; Uesugi S.-i.; Iwabuchi Y Org. Lett 2012, 14, 5006–5009. [DOI] [PubMed] [Google Scholar]; (i) Sonobe T; Oisaki K; Kanai M Chem. Sci 2012, 3, 3249–3255. [Google Scholar]; (j) Kim J; Stahl SS ACS Catal. 2013, 3, 1652–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Lauber MB; Stahl SS ACS Catal, in press (DOI: 10.1021/cs400746m). [DOI] [Google Scholar]
- (8).We have recently measured the redox potentials for each of the nitroxyls in Figure 1 under a uniform set of conditions. See ref 7k. A number of other nitroxyl redox potentials are reported in: Shibuya M; Pichierri F; Tomizawa M; Nagasawa S; Suzuki I; Iwabuchi Y Tetrahedron Lett. 2012, 53, 2070–2073. [Google Scholar]
- (9).As shown in refs 3d and 3i, Cu/TEMPO-catalyzed oxidation of activated alcohols benefit from electron-rich bpy derivatives. In contrast, Cu/TEMPO-catalyzed oxidation of aliphatic alcohols exhibit negligible bpy-based electron effects (see Figure S1 and Table S9).
- (10). When more challenging substrates were encountered, we first tested whether the reaction was successful at elevated temperatures (50–70 °C). If this approach failed to provide satisfactory results, we tested the use of pure O2 or the use of both elevated temperature and pure O2.
- (11).Aldehydes obtained from cis allylic alcohols are prone to alkene isomerization, even in the presence of a mild base. See ref 3i and the following: Clarke PA; Rolla GA; Cridland AP; Gill AA Tetrahedron 2007, 63, 9124–9128. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.