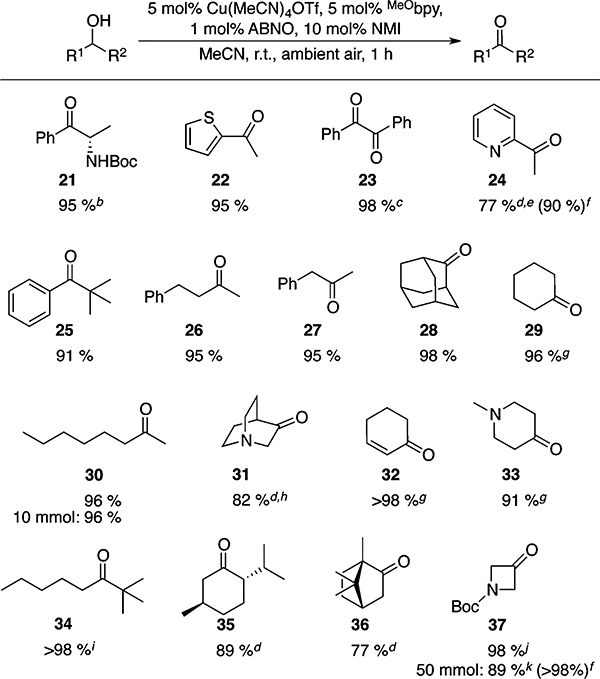
Table 2.
Scope of (MeObpy)CuI/ABNO-Catalyzed Aerobic Secondary Alcohol Oxidationa
Yields given are for isolated material. All reactions were performed on a 1 mmol scale in 10 mL of MeCN for 1 h in an open reaction vessel, unless otherwise noted.
>99% ee. 0.75 h reaction time.
Benzoin used as starting material.
Reaction performed at 70 °C with O2 balloon.10
2 h reaction time; 2 mol % catalyst added after 1.5 h (see SI for details).
1H NMR yield.
Isolated as the 2,4-dinitrophenylhydrazone due to product volatility.
Isolated as the HCl salt.
Reaction performed at 60 °C.10
0.5 reaction time.
0.2 M substrate concentration.