Abstract
Background
Hyperuricemia contributed to endothelial dysfunction, activation of the RAS system, increased oxidative stress and maladaptive immune system response. M1 and M2 macrophages were known to contribute to the onset of renal fibrosis. This study aimed to look at the effect of lowering serum uric acid levels on renal injury in mice.
Methods
This study used 25 male mice, 3 months old, that divided into 5 groups. We injected uric acid intraperitoneally, 125mg/kg/day for 7 days (UA7) and 14 days (UA14), to induce hyperuricemia and then gave allopurinol 50mg/kg/day for 7 days to lower serum uric acid levels (UA7AL7 and UA14AL7). At the end of the treatment, we measured serum uric acid levels, Glomerular Injury Score (GIS) and Arteriolar Injury Score (AIS) with PAS staining, eNOS and MCP-1 expression with Reverse Transcriptase-PCR (RT-PCR), macrophages M1/M2 ratio with anti-CD68 and anti-arginase I immunohistochemical staining. Data were analyzed by one-way ANOVA and Kruskal-Wallis test.
Results
Uric acid injection increased serum uric acid levels in UA7 and UA14 group (p<0.05), followed by the increase in GIS and AIS. RT-PCR showed increased expression of MCP-1 and decreased expression of eNOS. M1 macrophages count was higher than control in UA7 and UA14 whereas M2 macrophages did not show any increased count, so the ratio of macrophages M1 / M2 is higher. Decrease in serum uric acid levels reduced GIS, AIS, MCP-1 expression and macrophages M1/M2 ratio (p<0.05).
Conclusion
Reduction of serum uric acid levels significantly reduced renal injury that occurred in mice model of hyperuricemia.
Keywords: uric acid, allopurinol, renal injury, eNOS expression, MCP-1 expression, macrophage M1/M2 ratio
INTRODUCTION
The prevalence of hyperuricemia in population is high based on epidemiological studies in several countries [1–3]. Hyperuricemia is a cause of gout and urolithiasis because of formation and deposition of monosodium urate crystals. Hyperuricemia is a common finding in chronic kidney disease due to decreased clearance of uric acid[4]. Evidences have highlighted the role of uric acid as a cause or encourage the progression of cardiovascular disease and chronic kidney disease[5]. Increased serum uric acid level has an important role in insulin resistance and hypertension, which contributes to the emergence of cardiorenal metabolic syndrome, and cardiovascular disease associated with chronic kidney disease [6–8]. High serum uric acid level contributes to kidney injury due to inducing endothelial dysfunction with impairment of nitric oxide production [9–11], activation of the renin-angiotensin-aldosterone system[12], increased oxidative stress by NADPH Oxidase[13], and maladaptive immune system response by increased proinflammatory cytokines[14]. Those abnormalities will encourage fibrosis in vascular tissue, heart, and kidneys as well as associated functional abnormalities[6].
Allopurinol, a xanthine oxidase inhibitor, is a drug that conventionally used to decrease the synthesis of uric acid in the body[15]. Allopurinol inhibition to xanthine oxidase demonstrated anti-inflammatory effects on atherosclerosis, congestive heart failure, and acute lung injury. In addition, research shows renal injury caused by elevated levels of serum uric acid can be prevented by using allopurinol[16].
Macrophage traditionally has function as phagocyte that eliminates pathogen, apoptotic cell and cell debrises[17]. Beyond its traditional role in protecting the host from pathogens, macrophages play roles as a regulator of development, homeostasis, remodeling, and tissues repair. Macrophage undergoes polarization into different phenotypes, known as M1 and M2 macrophages, in response to external stimuli [18], and contributes to renal injury[19] and fibrosis[20]. However, there was little information about macrophage M1 and M2 involvement in hyperuricemic-induced renal injury. This study elucidates effects of uric acid levels reduction through allopurinol administration in renal injury and inflammation after uric acid treatment.
MATERIAL AND METHODS
Animal Subjects
Male Swiss mice 3 months old weighting 30 – 40 grams were acquired from Animal Model Care Unit, Gadjah Mada University. Mice were housed in animal facilities of Department of Anatomy, Faculty of Medicine, Universitas Gadjah Mada, Yogyakarta, Indonesia. Mice were housed under 12-hour of the natural light-dark cycle, humidity 50±5%, in plastic cages with 50×30×15cm in size, and with free access to standard food and water[21]. Mice were treated in compliance with the regulations and protocols of Medical and Health Research Ethics Committee (MHREC) of Faculty of Medicine, Universitas Gadjah Mada – Dr. Sardjito General Hospital (Forum for Ethical Review Committees in Asia and Western Pacific / FERCAP) for research involving animal. The study got ethics commitee approval from MHREC based on statement letter of ethical expediency no. KE/FK/1361/EC/2015 on December 2nd 2015. MHREC states that the research protocol meets the ethical principle outlined in the Declaration of Helsinki 2008.
Mice were adapted in the housing for 7 days prior to treatment. Mice were divided randomly into 5 groups ie control group, UA7 group, UA14 group, UA7AL7 group, and UA14AL7 group. This group divided according to previous research [20,22]. Sample size was calculated using Federer formula[23], which was 5 mice in each group so the total sample was 25 mice.
Mouse hyperuricemia model was generated by daily intraperitoneal injection of uric acid (U2625-25G, Sigma Aldrich) (125mg/kg), meanwhile NaCl 0.9% injection was used for control. Group of mice (n=5) were killed at 7 d (UA7 group) and 14 d (UA14 group). Another 2 group of mouse hyperuricemia model was generated. Blood uric acid levels was lowered using daily peroral allopurinol (A8003-25G, Sigma Aldrich, 50mg/kg) based on previous study[22]. Allopurinol was given for 7d (UA7AL7 group) and 14 d (UA14AL7 group) in hyperuricemic mice model. Uric acid dosage was refered to previous research and the results of optimization that has been done during this research. Dose of allopurinol was refered to previous research[24,25].
After the treatment was complete, mice was anesthetized with pentobarbital 1:10 (0.1 mL / 10gBB) via intraperitoneal injection[26]. Blood was collected from retroorbital vein for measuring serum levels of uric acid and creatinine. Abdomen and thorax were opened after deep anesthesia, and perfusion with NaCl 0.9% was performed via cardiac apex. Left kidney was harvested, put into RNA Later (7021, Ambion) solution for further RNA isolation. Right kidney was put into Normal Buffer Formaline (NBF) solution and then made into paraffin blocks.
Histologic examination of glomerular and arteriolar injury
Mouse kidney parrafin blocks were made into 4μm thick kidney sections. Kidney sections were stained with periodic acid-Schiff (PAS). Glomerular and arteriolar injuries were scored semi-quantitatively as described previously [27,28]. For evaluation of glomerular injury score, 20 glomeruli from each specimen were examined under light microscope (Olympus CX22®) and the image was captured using the Optilab software in 400× of magnification. Each glomerulus was scored as follows: score 0 = normal glomerulus; score 1 = damage < 1/3 glomerular area; score 2 = damage 1/3 – 2/3 glomerular area; score 3 = damage > 2/3 to entire area of the glomerulus. Glomerular damage was defined as sclerosis deposition, capillary tuft closing and adhesion of glomerulus to Bowman’s capsule. For evaluation of arteriolar injury score, 20 glomeruli from each specimen were examined and were scored as follows: score 0 = normal arterioles; 1 = hyalinosis of <50% arteriolar wall circumference; score 2 = hyalinosis of 50–100% arteriolar wall circumference without luminal narrowing; score 3 = hyalinosis of 100% arteriol wall circumference with luminal narrowing of arteriol.
Imunohistochemical (IHC) staining
Paraffin embedded sections were stained with anti-CD68 (ab955, Abcam) for pan-macrophages detection and anti-Arginase I (sc-20150, Santa Cruz Biotechnology) for M2 macrophages detection. IHC staining was completed using Starr Trek Universal HRP Detection Kit (STUHRP700Hkit, Biocare Medical).
Briefly, after deparaffinization, antigen retrieval with heating method using citrate buffer for 15 minutes was used, continued by inhibition of peroxidase with H2O2 3% for 5 minutes. Slides were incubated in 1% BSA in PBS for unspecific blocking, continued by incubation of first antibodies: anti-CD68 antibody (1: 400) and anti-Arginase I antibody (1: 500) for overnight at 4oC. Secondary antibody was applied using Trekkie Universal Link 1–2 drops for 60 minutes, then Trek Avidin HRP label 1–2 drops for 45 minutes. DAB labeling was done, continued by counter staining with hematoxylin.
Slide viewed with light microscope (Olympus CX22®) and the image was captured using the Optilab software. CD68 and Arginase I positive cell numbers per high-power field (×400) were counted. Ten randomly selected field of each kidney were counted. Macrophage M1/M2 ratio was calculated according to previous research[29] with a modification. M1 macrophages count calculated by CD68 positive cell count (pan-macrophages count) – Arginase I positive cell count (M2 macrophages count). The M1/M2 ratio calculated by dividing M1 macrophages count / M2 macrophages count.
RNA extraction, cDNA synthesis and Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
Total RNA was extracted from 50–100 mg of the left kidney tissue using GENEzol™ (GZR100, Geneaid) according to the instructions by the manufacturer. RNA concentration was quantified using a spectrophotometer. cDNA was synthesized from 3μg of total RNA using ReverTra Ace® kit according to the instructions by the manufacturer (Toyobo, TRT 1001).
PCR was performed using GoTaq® Green Master Mix (M7122, Promega) on 2 μl aliquots of cDNA with spesific primer pairs. The sequences of primer pairs as follows: Monocyte Chemoattractant Protein-1 (MCP-1) (forward: 5′-CTA CAG ACA AGC TCA ACC ACC ACT TCT GTA G-3′; reverse: 5′-GGC ATC ACA ACA GTC GTC CGA C-3′), endothelial Nitrite Oxydase Synthase (eNOS) (forward: 5′-GTC GTG CAG CTG CAA ACC AG-3′; reverse: 5′-TGG GTG CGC GTG AAT AGT C-3′), and Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH) (forward: 5′-TGT GTC CGT CGT GGA TCT GA-3′; reverse: 5′-AAG TTG TTG CTG TCG CAG GAG-3′). PCR conditions as follows: initial denaturation 94°C for 2 min, 30 cycles with 94°C for 10 seconds, 60°C for 30 seconds and 72°C for 1 minute, final extension phase 72°C for 10 minutes. The PCR products were size fractioned on a 2% agarose gel and detected by GelRed™ (41003, Biotium) staining. Quantification was performed by measurement of the intensity of the signals with aid of Image J software.
Statistical analysis
Statistical analysis was performed using SPSS 22 software for windows. Comparison between groups were made using one way ANOVA test and post hoc LSD test if data were normally distributed. If data were not distributed normally then data analized with Kruskal-Wallis test and post hoc Mann-Whitney test. P <0.05 was considered significant.
RESULTS
Reduction of uric acid in allopurinol groups associated with reduction of creatinine, glomerular injury score and arteriolar injury score
Uric acid treatment in mice induced increased serum uric acid levels after 7 and 14 days of treatment, the same as shown by our previous result[30]. Then, we administered allopurinol for reducing serum uric acid levels in mice. Figure 1B and 1C showed that allopurinol which was given to UA7AL7 and UA14AL7 group lowered serum uric acid levels and serum creatinine levels was lowered as well.
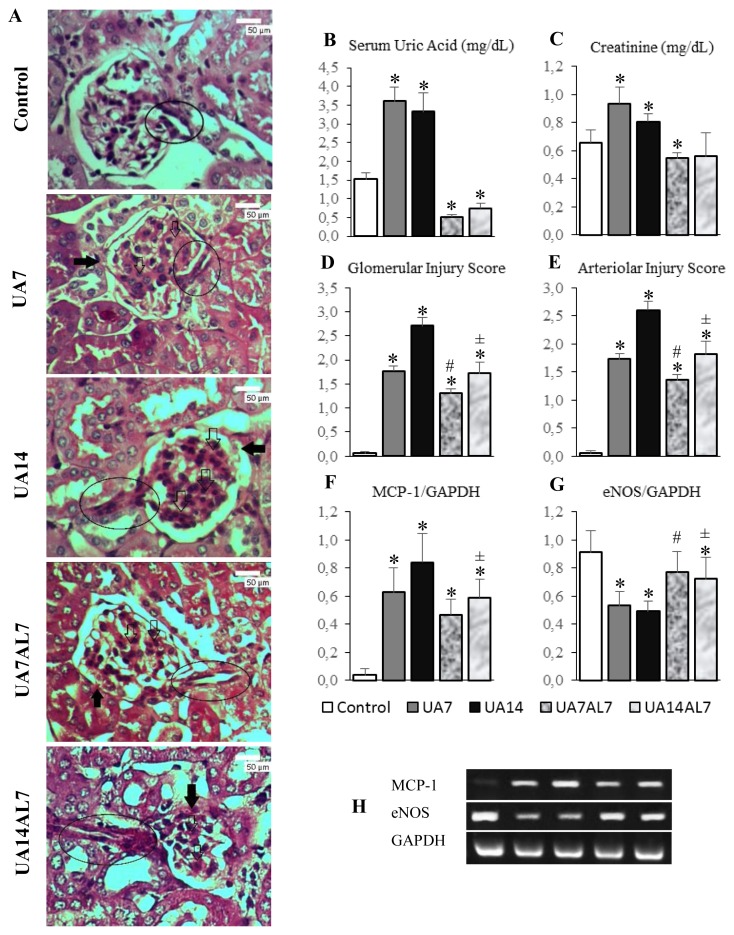
Figure 1.
(A) Histopathologic examination of mice kidneys with PAS staining, light microscope of 400 times magnification. Note: (➔) synechiae, (⇨) glomerulosclerosis, inside oval box: arteriole. Glomerular damage occured which was characterized by glomerulosclerosis, synechiae, and thickening of the basement membrane in all groups except the control. Glomerulosclerosis occured most severe in UA14 group. Afferent arteriolar was damaged in the form of narrowing lumen and hyalinosis. The most severe damage was on the UA14 group, where there was total obliteration of the lumen of the afferent arterioles. (B) Serum uric acid levels bar chart. (C) Serum creatinine levels. (D) Glomerular injury score bar chart. (E) Arteriolar injury score bar chart. (F) MCP-1 gene expression bar chart. (G) eNOS gene expression bar chart. (H) MCP-1, eNOS and GAPDH gene expression electrophoresis band. * = p <0.05 vs Control; # = p <0.05 vs UA7; ± = p <0.05 vs UA14
Hyperuricemia (UA7 and UA14 groups) caused glomerular injury in mouse kidney, characterized by sclerosis, synechiae and thickening of the basement membrane. Kidney arteriolar also damaged in form of narrowing lumen and hyalinosis (Figure 1A). When serum uric acid levels was lowered (UA7AL7 and UA14AL7 groups), the damage was ameliorated (Figure 1A). Semiquantitative analysis of both glomerular and arteriolar injuries showed reduction of both injuries in mice with allopurinol (Figure 1D and 1E).
Hyperuricemia decreased eNOS expression in mouse kidney
Further, we examined eNOS expression to confirm reduction of vasodilator agent which might contribute to arteriol injury. Hyperuricemia groups (UA7 and UA14 groups) had significant reduction of eNOS expression compared to control. Meanwhile, administration of allopurinol after hyperuricemia condition increased eNOS expression significantly (Figure 1G). We did not found significant difference in eNOS expression between control and UA7AL7 group).
Hyperuricemia cause inflammation in kidney
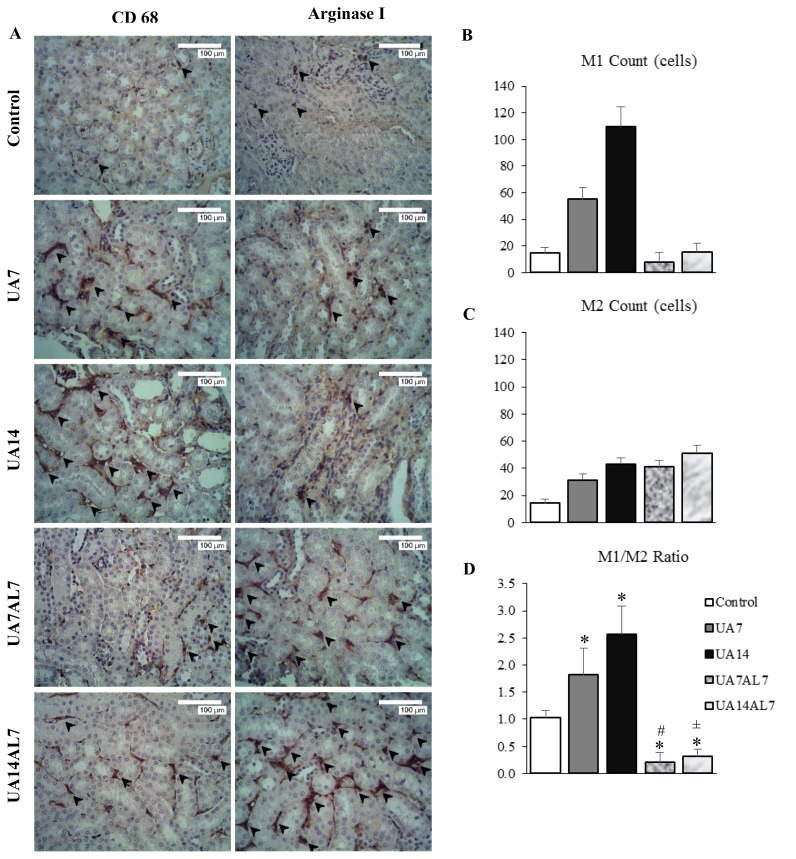
We examined the expression of MCP-1 in mouse kidney to assess inflammation. Hyperuricemia increased MCP-1 expression in mouse kidney compared to control (Figure 1F). We stained kidney sections with anti-CD68 and anti-Arginase I for assesment of macrophage infiltration in mouse kidney (Figure 2A). Increased expression of MCP-1 was accompanied with macrophage infiltration in tubulointerstitial region of mouse kidney. In mouse hyperuricemia model, M1 macrophage count (Figure 2B) were higher than M2 macrophage count (Figure 2C), resulted in higher M1/M2 ratio (Figure 2D). When we lowered serum uric acid levels using allopurinol, the M1/M2 ratio was lowered as well.
Figure 2.
(A) Immunohistochemical staining of CD68 and arginase I antibodies, observed with light microscope of 400 times magnification. Brown stained cells which showed by arrow were macrophage cells. Macrophages that express CD68 highest were in the UA14 group. Macrophages that express arginase I highest were in the UA14AL7 group. (B) Macrophage M1 count bar chart. (C) Macrophage M2 count bar chart. (D) Macrophage M1/M2 ratio bar chart. * = p <0.05 vs Control; # = p <0.05 vs UA7; ± = p <0.05 vs UA14
DISCUSSION
This study was continuation of our previous study, which uric acid 125mg/kg/day intraperitoneal injection induced hyperuricemia condition with elevation of serum uric acid levels[30]. Furthermore, we administered allopurinol orally after 7 and 14 days of uric acid injection for 7 days. Allopurinol administration reduced serum uric acid levels in both of 7 and 14 days uric acid injection groups (UA7AL7 and UA14AL7 groups) as shown in figure 1B. We also examined serum creatinine levels and found that it increased associated to serum uric acid levels in UA7 and UA14 groups. Meanwhile, groups of mice that were given allopurinol (UA7AL7 and UA14AL7) showed their serum uric acid levels were significantly lower than the control group. This suggests allopurinol 50 mg/kg/day can lower serum uric acid levels in hyperuricemic mice. Normal uric acid levels in mice ranged from 0.5 to 1.5 mg / dL [31]. These results are consistent with another previous reports[24,25]. This result showed a decrease in serum uric acid levels has a beneficial effect on the kidney function and consistent with previous study[32] that obtained results of allopurinol administration reduces serum creatinine levels in hyperuricemic mice.
All groups had glomerular and arteriolar injury score higher than the control, showed the damage caused by hyperuricemia. In the other hand, UA7AL7 group glomerular injury score lower than UA7 group, showed improvements in glomerular damage when blood uric acid levels lowered. The same thing also seen on the score of UA14AL7 group glomerular injury score compared to UA14 group. These results are consistent with the results of recent study[33] that obtained results of hyperuricemia caused renal glomerulosclerosis, while allopurinol lower glomerular injury that occurred.
Previous investigation showed that hyperuricemic mice have hypertension and afferent arterioles thickening. The study also showed that the thickening of the afferent arterioles mediated by a direct effect of uric acid on the proliferation of vascular smooth muscle cells by activation of RAS[34]. Another study also showed that hyperuricemia accelerate renal injury by enhancing expression of renin in the renal cortex and expression of cyclooxygenase-2 (COX-2) in the afferent arterioles[35]. Uric acid cause activation of MAPK and NF-kB on smooth muscle of arterioles [36] and tubular epithelial cell that increases the expression of proinflammatory cytokines MCP-1 thus encourage infiltration of macrophages into the interstitial renal[22]. These macrophages then will produce cytokines such as TNF-α and MCP-1 that induced inflammatory process in kidney tissue and infiltration of more macrophages[37].
Arteriolar injury in UA7 and UA14 groups might associated with significant downregulation of eNOS expression in those groups. In this study, the UA7 and UA14 group has a significantly lower eNOS expression compared to control, UA7AL7 and UA14AL7 group, indicated endothelial dysfunction in hyperuricemia (Figure 1G). eNOS expression of UA7AL7 and UA14AL7 group were significantly higher than UA7 and AL14, showed an improvement in endothelial function after serum uric acid levels lowered. Uric acid induced endothelial dysfunction as a result of oxidative stress. Previous study showed that in a mouse model of OA-induced hyperuricemia, intrarenal hyperuricemia causes oxidative stress, increased expression of NOX-4 subunit of NADPH oxidase and angiotensin II, and decreased bioavailability of nitric oxide (NO)[13]. Subsequent research had reported that endothelial dysfunction induced by uric acid is associated with changes in the mitochondria and decreased intracellular ATP concentration. Uric acid causes a decrease in the expression of the enzyme aconitase-2 and enoyl CoA hydratase-1 in the mitochondria. There was also an increase in mitDNA damage and decrease in mitochondria mass in endothelial cells[38].
Uric acid induces inflammation process, especially in kidney. In vitro study using renal tubular epithelial cells showed increased MCP-1 expression after administration of uric acid[22]. This study also revealed upregulation of MCP-1 expression in UA7 and UA14 groups compared to control. Reduction of serum uric acid levels in UA14AL7 group might associate with significantly decreased MCP-1 expression compared to UA14 group. Focusing on the M1 and M2 macrophage polarization, we did IHC staining of anti-CD68 and anti-Arginase I antibodies. The M1/M2 ratio was quantified based on the IHC. This research obtained results UA7 and UA14 group had significantly higher macrophage M1/M2 ratio compared to control, UA7AL7 and UA14AL7 group (Figure 2D). It indicated when serum uric acid levels was high, the number of M1 macrophages was high while the number of M2 macrophages was low. Furthermore, when the serum uric acid levels decreased (UA7AL7 and UA14AL7 groups), the number of M1 macrophages decreased while the number of M2 macrophages increased. This resulting in significantly lower macrophage M1/M2 ratio compared to control which showing improvement in allopurinol given group.
A study showed that uric acid induced TNF-α expression in renal tubular epithelial cells[22]. Uric acid can gain entry into the macrophages where it interacts with NLRP3 inflammasome to produce IL-1β. The IL-1β then attracts macrophages to the kidney[39]. TNF-α will induce macrophages polarization into M1 form. The M1 macrophages then produce more TNF-α, MCP-1 and IL-6 that further recruit more macrophages and induce more macrophages to polarize into M1 form[40,41]. Uric acid can also directly activate Toll-like receptors (TLR), especially TLR2/TLR4, in macrophages which activate NF-kB pathway that drive the macrophage into M1 form[40].
When serum uric acid levels decreased, a decrease in the number of M1 macrophages may occur due to M1 macrophages polarized to M2 which has the function of tissue repair. A recent study with a mouse model of ischemia-reperfusion, obtained results that the failure of macrophages to switch from proinflammatory phenotype M1 to M2 can encourage inflammation and progressive renal fibrosis, even after resolution of ischemia-reperfusion injury[19]. On the other hand, macrophages M2 also has a profibrotic effect. M2 Macrophages can release insulin-like growth factor-1 (IGF-1), fibroblast growth factor-2 (FGF-2) and platelet-derived growth factor (PDGF), which promotes myofibroblast proliferation [42]. Another recent study showed the macrophages have an important role in the onset of renal fibrosis. This study, using a mouse model of UUO (unilateral ureteral obstruction), got the results that M1 subtype of macrophage infiltrated in the kidney after acute kidney injury. Along with the passage of renal injury, macrophages polarized to the M2 subtype[20].
Based on the results obtained in the study, it can be concluded hyperuricemic mice that were given allopurinol had significantly lower glomerular injury score, arteriolar injury score, MCP-1 expression, and macrophages M1/M2 ratio compared to hyperuricemic mice model. eNOS expression in hyperuricemic mice that were given allopurinol were significantly higher than hyperuricemic mice.
ACKNOWLEDGEMENTS
This study was funded by Penelitian Unggulan Perguruan Tinggi (PUPT) and Lembaga Pengelola Dana Pendidikan (LPDP). The authors would like to thank Mr. Mulyana for technical support for the animal experiment, Wiwit Ananda Wahyu and Hayu Qaimamunazzala for assisting in RT-PCR and IHC procedure. This research had been used for finishing Master Degree.
REFERENCES
- 1.Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: The National Health and Nutrition Examination Survey 2007–2008. Arthritis Rheum. 2011;63(10):3136–41. doi: 10.1002/art.30520. [DOI] [PubMed] [Google Scholar]
- 2.Hakoda M. Recent trends in hyperuricemia and gout in Japan. Japan Med Assoc J. 2012;55(4):319–23. [PubMed] [Google Scholar]
- 3.Liu B, Wang T, Zhao H, Yue W, Yu H, Liu C, et al. The prevalence of hyperuricemia in China: a meta-analysis. BMC Public Health. 2011;11(1):832. doi: 10.1186/1471-2458-11-832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Filiopoulos V, Hadjiyannakos D, Vlassopoulos D. New insights into uric acid effects on the progression and prognosis of chronic kidney disease. Ren Fail. 2012;34:510–20. doi: 10.3109/0886022X.2011.653753. [DOI] [PubMed] [Google Scholar]
- 5.Gustafsson D, Unwin R. The pathophysiology of hyperuricaemia and its possible relationship to cardiovascular disease, morbidity and mortality. BMC Nephrol. 2013;14(1):1. doi: 10.1186/1471-2369-14-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaudhary K, Malhotra K, Sowers J, Aroor A. Uric acid-key ingredient in the recipe for cardiorenal metabolic syndrome. CardioRenal Med. 2013;3(3):208–20. doi: 10.1159/000355405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iliesiu A, Campeanu A, Dusceac D. Serum uric acid and cardiovascular disease. Mædica. 2010;5(3):186–92. [PMC free article] [PubMed] [Google Scholar]
- 8.Zoccali C, Maio R, Mallamaci F, Sesti G, Perticone F. Uric acid and endothelial dysfunction in essential hypertension. J Am Soc Nephrol. 2006;17(5):1466–71. doi: 10.1681/ASN.2005090949. [DOI] [PubMed] [Google Scholar]
- 9.Papežíková I, Pekarová M, Kolářová H, Klinke A, Lau D, Baldus S, et al. Uric acid modulates vascular endothelial function through the down regulation of nitric oxide production. Free Radic Res. 2013;47(2):82–8. doi: 10.3109/10715762.2012.747677. [DOI] [PubMed] [Google Scholar]
- 10.Choi YJ, Yoon Y, Lee KY, Hien TT, Kang KW, Kim KC, et al. Uric acid induces endothelial dysfunction by vascular insulin resistance associated with the impairment of nitric oxide synthesis. FASEB J. 2014;28(7):3197–204. doi: 10.1096/fj.13-247148. [DOI] [PubMed] [Google Scholar]
- 11.Kanbay M, Yilmaz MI, Sonmez A, Turgut F, Saglam M, Cakir E, et al. Serum uric acid level and endothelial dysfunction in patients with nondiabetic chronic kidney disease. Am J Nephrol. 2011;33(4):298–304. doi: 10.1159/000324847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mazzali M, Hughes J, Kim YG, Jefferson JA, Kang DH, Gordon KL, et al. Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension. 2001;38(5):1101–6. doi: 10.1161/hy1101.092839. [DOI] [PubMed] [Google Scholar]
- 13.Sánchez-Lozada LG, Soto V, Tapia E, Avila-Casado C, Sautin YY, Nakagawa T, et al. Role of oxidative stress in the renal abnormalities induced by experimental hyperuricemia. Am J Physiol Renal Physiol. 2008;295(4):F1134–41. doi: 10.1152/ajprenal.00104.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baldwin W, Mcrae S, Marek G, Wymer D, Pannu V, Baylis C, et al. Hyperuricemia as a mediator of the proinflammatory endocrine imbalance in the adipose tissue in a murine model of the metabolic syndrome. Diabetes. 2011;60(4):1258–69. doi: 10.2337/db10-0916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pacher P, Nivorozhkin A, Szabo C. Therapeutic effects of xanthine oxidase inhibitors: Renaissance half a century after the discovery of allopurinol. Hum Physiol. 2006;58(1):87–114. doi: 10.1124/pr.58.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim IY, Lee DW, Lee SB, Kwak IS. The role of uric acid in kidney fibrosis: Experimental evidences for the causal relationship. Biomed Res Int. 2014;2014(638732):1–9. doi: 10.1155/2014/638732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11(11):723–37. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stefater JA, Ren S, Lang RA, Duffield JS. Metchnikoff’s policemen: Macrophages in development, homeostasis and regeneration. Trends Mol Med. 2011;17(12):743–52. doi: 10.1016/j.molmed.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lech M, Gröbmayr R, Ryu M, Lorenz G, Hartter I, Mulay SR, et al. Macrophage phenotype controls long-term aki outcomes -- kidney regeneration versus atrophy. J Am Soc Nephrol. 2013;25:1–13. doi: 10.1681/ASN.2013020152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pan B, Liu G, Jiang Z, Zheng D. Regulation of renal fibrosis by macrophage polarization. Cell Physiol Biochem. 2015;35(3):1062–9. doi: 10.1159/000373932. [DOI] [PubMed] [Google Scholar]
- 21.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving Bioscience Research Reporting: The ARRIVE Guidelines for Reporting Animal Research. PLoS Biol. 2010;8(6):e1000412. doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou Y, Fang L, Jiang L, Wen P, Cao H, He W, et al. Uric acid induces renal inflammation via activating tubular NF-κB signaling pathway. PLoS One. 2012;7(6):1–10. doi: 10.1371/journal.pone.0039738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.David D, Arkeman H. Evaluation of the oral toxicity of formaldehyde in rats. Universa medicina. 2008;27(3):106–112. [Google Scholar]
- 24.Peglow S, Toledo AH, Anaya-Prado R, Lopez-Neblina F, Toledo-Pereyra LH. Allopurinol and xanthine oxidase inhibition in liver ischemia reperfusion. J Hepatobiliary Pancreat Sci. 2011;18(2):137–46. doi: 10.1007/s00534-010-0328-7. [DOI] [PubMed] [Google Scholar]
- 25.Derbre F, Ferrando B, Gomez-Cabrera MC, Sanchis-Gomar F, Martinez-Bello VE, Olaso-Gonzalez G, et al. Inhibition of xanthine oxidase by allopurinol prevents skeletal muscle atrophy: Role of p38 MAPKinase and E3 Ubiquitin Ligases. PLoS One. 2012;7(10):1–9. doi: 10.1371/journal.pone.0046668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leary S, Underwood W, Lilly E, Anthony R, Cartner S, Corey D, et al. AVMA guidelines for the euthanasia of animals: 2013 edition. American Veterinary Medical Association; 2013. pp. 18–41. [Google Scholar]
- 27.Zhou XJ, Vaziri ND, Zhang J, Wang HW, Wang XQ. Association of renal injury with nitric oxide deficiency in aged SHR: Prevention by hypertension control with AT1 blockade. Kidney Int. 2002;62(3):914–21. doi: 10.1046/j.1523-1755.2002.00516.x. [DOI] [PubMed] [Google Scholar]
- 28.Klopfleisch R. Multiparametric and semiquantitative scoring systems for the evaluation of mouse model histopathology--a systematic review. BMC Vet Res. 2013;9(1):123. doi: 10.1186/1746-6148-9-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang M, He Y, Sun X, Li Q, Wang W, Zhao A, et al. A high M1/M2 ratio of tumor-associated macrophages is associated with extended survival in ovarian cancer patients. J Ovarian Res. 2014;7(1):19. doi: 10.1186/1757-2215-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Setyaningsih W, Arfian N, Suryadi E, Romi M, Tranggono U, Sari D. Hyperuricemia induces Wnt5a / Ror2 gene expression, epithelial – mesenchymal ttransition (EMT), and kidney tubular injury in mice. Iran J Med Sci. 2017;41(2):1–10. [PMC free article] [PubMed] [Google Scholar]
- 31.Watanabe S, Kang DH, Feng L, Nakagawa T, Kanellis J, Lan H, et al. Uric acid, hominoid evolution, and the pathogenesis of salt-sensitivity. Hypertension. 2002;40(3):355–60. doi: 10.1161/01.hyp.0000028589.66335.aa. [DOI] [PubMed] [Google Scholar]
- 32.Ryu ES, Kim MJ, Shin HS, Jang YH, Choi HS, Jo I, et al. Uric acid-induced phenotypic transition of renal tubular cells as a novel mechanism of chronic kidney disease. Am J Physiol Renal Physiol. 2013;304(5):F471–80. doi: 10.1152/ajprenal.00560.2012. [DOI] [PubMed] [Google Scholar]
- 33.Diwan V, Mistry A, Gobe G, Brown L. Adenine-induced chronic kidney and cardiovascular damage in rats. J Pharmacol Toxicol Methods. 2013;68(2):197–207. doi: 10.1016/j.vascn.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 34.Mazzali M, Kanellis J, Han L, Feng L, Xia YY, Chen Q, et al. Hyperuricemia induces a primary renal arteriolopathy in rats by a blood pressure-independent mechanism. Am J Physiol Ren Physiol. 2002;282(6):F991–7. doi: 10.1152/ajprenal.00283.2001. [DOI] [PubMed] [Google Scholar]
- 35.Kang DH, Nakagawa T, Feng L, Watanabe S, Han L, Mazzali M, et al. A role for uric acid in the progression of renal disease. J Am Soc Nephrol. 2002;13(12):2888–97. doi: 10.1097/01.asn.0000034910.58454.fd. [DOI] [PubMed] [Google Scholar]
- 36.So A, Thorens B. Uric acid transport and disease. J Clin Invest. 2010;120(6):1791–9. doi: 10.1172/JCI42344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Billiet L, Doaty S, Katz JD, Velasquez MT. Review of hyperuricemia as new marker for metabolic syndrome. ISRN Rheumatol. 2014;2014(852954):1–7. doi: 10.1155/2014/852954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sánchez-Lozada LG, Lanaspa MA, Cristóbal-García M, García-Arroyo F, Soto V, Cruz-Robles D, et al. Uric acid-induced endothelial dysfunction is associated with mitochondrial alterations and decreased intracellular ATP concentrations. Nephron - Exp Nephrol. 2013;121(3–4):71–8. doi: 10.1159/000345509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Braga TT, Forni MF, Correa-Costa M, Ramos RN, Barbuto JA, Branco P, et al. Soluble uric acid activates the NLRP3 inflammasome. Sci Rep. 2017;7:1–14. doi: 10.1038/srep39884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ghaemi-Oskouie F, Shi Y. The role of uric acid as an endogenous danger signal in immunity and inflammation. Curr Rheumatol Rep. 2011;13(2):160–6. doi: 10.1007/s11926-011-0162-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martin WJ, Shaw O, Liu X, Steiger S, Harper JL. Monosodium urate monohydrate crystal-recruited noninflammatory monocytes differentiate into M1-like proinflammatory macrophages in a peritoneal murine model of gout. Arthritis Rheum. 2011;63(5):1322–32. doi: 10.1002/art.30249. [DOI] [PubMed] [Google Scholar]
- 42.Meng XM, Nikolic-Paterson DJ, Lan HY. Inflammatory processes in renal fibrosis. Nat Rev Nephrol. 2014;10(9):493–503. doi: 10.1038/nrneph.2014.114. [DOI] [PubMed] [Google Scholar]