Abstract
Pericytes and glial cells are accessory cells of neurovascular networks, which have been reported to participate in scar formation after tissue injury. However, it remains unclear whether similar reactive cellular responses occur in pancreatic intraepithelial neoplasia (PanIN). In this study we developed three-dimensional (3D) duct lesion histology to investigate PanIN and the associated pericyte, glial, and islet remodeling. Transparent mouse pancreata with a KrasG12D mutation were used to develop 3D duct lesion histology. Deep-tissue, tile-scanning microscopy was performed to generate panoramic views of the diseased pancreas for global examination of early stage and advanced duct lesion formation. Fluorescence signals of ductal and neurovascular networks were simultaneously detected to reveal associated remodeling. Significantly, in KrasG12D-mutant mice, when the low-grade PanINs emerge, duct lesions appear as epithelial buds with perilesional pericyte and glial activation. When PanINs occur in large scale (induced by cerulein injections to the mutant mice), the 3D image data identifies 1) aggregation of PanINs in clusters in space; 2) overexpression of the pericyte marker NG2 in the PanIN microenvironment; and 3) epithelial in-growth to islets, forming the PanIN-islet complexes. Particularly, the PanIN-islet complexes associate with proliferating epithelial and stromal cells and receive substantial neurovascular supplies, making them landmarks in the atrophic lobe. Overall, perilesional pericyte and glial activation and formation of the PanIN-islet complex underline cellular heterogeneity in the duct lesion microenvironment. The results also illustrate the advantage of using 3D histology to reveal previously unknown details of neurovascular and endocrine links to the disease.
Keywords: 3D histology, KrasG12D mutation, optical clearing, pancreatic intraepithelial neoplasia, PanIN-islet complex
NEW AND NOTEWORTHY
Transparent mouse pancreata with an acinar KrasG12D mutation were prepared by optical clearing to develop 3D duct lesion histology. This imaging approach identifies perilesional pericyte and glial activation and formation of pancreatic intraepithelial neoplasia (PanIN)-islet complexes, which otherwise cannot be easily portrayed by standard 2D tissue analysis. Our results highlight the cellular heterogeneity in the PanIN microenvironment and reveal anatomic details of neurovascular and endocrine links to the disease.
the pancreas consists of acini and islets in connection with the ductal and neurovascular networks to perform exocrine and endocrine functions. Morphologically, the tree-like ductal epithelium occupies the central position in the pancreas, which during development gives rise to the exocrine and endocrine cells and forms the ductal network to transport the pancreatic enzymes (17). In disease, the progression of pancreatic duct lesions is a process of transformation and remodeling of the ductal epithelium, ranging from formation of low-grade pancreatic intraepithelial neoplasia (PanIN) lesions to metastatic adenocarcinoma (22). This process is often associated with the oncogenic Kras mutation and pancreatitis, which in animals, has been shown to induce transformation of exocrine acinar cells to duct-like epithelial cells and unregulated epithelial and stromal proliferation (1, 10, 19, 28, 38). To date, staging of duct lesions, the PanIN system, has been well established to morphologically characterize epithelial remodeling (22). However, how the progression of diseased epithelium influences the pancreatic neurovascular networks and endocrine islets is incompletely understood.
Pathophysiologically, the perilesional neurovascular networks link the duct lesion microenvironment to pancreatic circulation and nervous system, which provide nutrients and stimuli to support/induce epithelial outgrowth and stromal development. In the pancreas, the neural tissues include the autonomic nerves (sympathetic, parasympathetic, and sensory nerves) and the glial network (2, 3, 11). The glial network is formed by glial cells (or Schwann cells), which release neurotrophic factors such as the nerve growth factor, and the glial cell line-derived neurotrophic factor to support the nerves and islets (31, 41). For example, mouse glial cells form a sheath in the mantle of an islet to envelop the neuroendocrine tissue (39). In the pancreatic vasculature, blood vessels consist of endothelium and pericytes (or mural cells) (24, 34). The endothelium recruits pericytes through the release of the platelet-derived growth factor to establish physical contact from the abluminal direction to stabilize the vascular system (27, 43). Importantly, although pericytes and glial cells have been considered accessory cells to support the neurovascular system, studies of the two cell types have indicated their reactivities in response to tissue injury and inflammation.
First, in rodent models of pancreatic islet injury induced by lymphocytic invasion or streptozotocin injection, both pancreatic glial cells and pericytes become reactive following islet lesion formation and vascular damage (40, 41). Second, in an in vitro assessment of the microenvironment in chronic pancreatitis and pancreatic cancer, human tissue extracts derived from both conditions induce neural tissue outgrowth, including an increase in glial cell density (9). Third, studies of renal pericytes have linked pericyte activation to fibrosis after renal injury (6, 23). Fourth, after injury to the central nervous system, both glial cells and pericytes are activated to form a glial scar around the lesion (13, 18).
On the basis of these reported reactivities of pericytes and glial cells, a study of pancreatic duct lesions ideally should include the two cell types to examine their roles in affecting the microvessels and innervation. From a therapeutic perspective, analysis of the neurovascular tissues around the duct lesions provides an opportunity to understand the pathway or to avoid potential barriers for therapeutic agents to reach the stroma and lesion for effective therapy. However, high-definition image analysis of neurovascular tissues in the lesion microenvironment is lacking. This is largely a result of the dispersed nature of the ductal and neurovascular networks, which cannot be easily portrayed via standard microtome-based two-dimensional (2D) histology to characterize their spatial relationship.
In this research, transparent mouse pancreata with acinar KrasG12D mutation are prepared by optical clearing (7, 8, 15) to develop 3D duct lesion histology with the resolving power of revealing associated ductal and neurovascular remodeling. Qualitative and quantitative analyses of pancreatic duct lesions, perilesional pericyte and glial activation, and unexpected formation of the PanIN-islet complex are presented and discussed.
MATERIALS AND METHODS
Animals.
Pancreata harvested from 14-wk-old elastase-CreER-KrasG12D mice (elastase-CreER × LSL-KrasG12D) (42, 44) were used to develop 3D duct lesion histology. To examine early stage duct lesion formation, elastase-CreER-KrasG12D mice were injected with tamoxifen (Sigma, St. Louis, MO) at age 6 wk (2 mg/injection, three injections in 1 wk to induce Cre-mediated recombination), and duct lesion was allowed to develop for the next 7 wk. To examine advanced duct lesion formation, elastase-CreER-KrasG12D mice were injected with tamoxifen at age 6 wk and then with cerulein (Sigma) at age 7 wk (0.25 mg/kg body wt, six injections per week for 3 consecutive wk) to induce large-scale development of PanINs (PanIN-1/2). To examine duct lesion formation in chronic pancreatitis, wild-type C57BL/6 (B6) mice (National Laboratory Animal Center, Taipei, Taiwan) were injected with cerulein at age 7 wk (six injections per week for 6 consecutive wk) to induce epithelial overgrowth. Overall, four animals under each condition were used to generate representative images. The Institutional Animal Care and Use Committees at National Tsing Hua University and Academia Sinica approved all procedures with mice.
Preparation of pancreatic specimens.
Pancreatic blood vessels were labeled with cardiac perfusion of lectin-Alexa Fluor 488 conjugates [30 μg/g of body wt, Invitrogen, Carlsbad, CA (these fluorescent dyes bind to the endothelial cellular membranes in perfusion, a process known as vessel painting) (33)] followed by 4% paraformaldehyde perfusion fixation. Afterward, the pancreas was harvested and postfixed in 4% paraformaldehyde solution for 40 min at 15°C. Vibratome sections of fixed tissue (∼400 μm) were then immersed in 2% Triton X-100 solution for 2 h at 15°C for permeabilization.
Six different primary antibodies were used to immunolabel tissues following the protocol outlined below. Antibodies that were used included rabbit anti-cytokeratin 19 (CK19, a duct epithelial marker, ab133496; Abcam, Cambridge, MA), rabbit anti-neuron-glial antigen 2 (NG2, a pericyte marker, AB5320; Millipore, Billerica, MA), rabbit antiglial fibrillary acidic protein (GFAP, a glial marker, Z0334; DAKO, Carpinteria, CA), rabbit anti-TUJ1 (a neuron-specific class III β-tubulin neuronal marker, PRB-435P; Covance, Princeton, NJ), rabbit anti-Ki-67 (a nuclear protein associated with cellular proliferation, ab15580; Abcam), and guinea pig anti-insulin (GTX27842; GeneTex, Irvine, CA). Before applying the antibody, the tissue was rinsed in PBS. This was followed by a blocking step that entailed incubating the tissue with blocking buffer (2% Triton X-100, 10% normal goat serum, and 0.02% sodium azide in PBS). The primary antibody was then diluted in dilution buffer (1:100, 0.25% Triton X-100, 1% normal goat serum, and 0.02% sodium azide in PBS) to replace the blocking buffer and incubated for 1 day at 15°C.
Alexa Fluor 647-conjugated goat anti-rabbit and goat anti-guinea pig secondary antibodies (1:200, Invitrogen) were used to reveal immunostained structures. Nuclear staining with propidium iodide (50 μg/ml, Invitrogen) was performed at room temperature for 1 h to reveal nuclei. Labeled specimens were then immersed in tissue-clearing solution (RapiClear 1.52 solution, SunJin Lab, Hsinchu, Taiwan) before being imaged via confocal microscopy (7, 14).
Deep-tissue confocal microscopy.
Imaging of the tissue structure was performed with a Zeiss LSM 510 Meta confocal microscope (Carl Zeiss, Jena, Germany) equipped with ×10 Fluar lenses, ×25 LD Plan-Apochromat lenses (working distance 570 μm), and ×40 LD C-Apochromat lenses (working distance 620 μm) under a tile-scan mode with automatic image stitching. The laser-scanning process was operated under the multitrack scanning mode to sequentially acquire signals, including transmitted light signals. Alexa Fluor 647-labeled structures were excited at 633 nm, and fluorescence was collected with a band-pass filter (650–710 nm). Propidium iodide-labeled nuclei were excited at 543 nm, and signals were collected with a band-pass filter (560–615 nm). Lectin-Alexa Fluor 488-labeled blood vessels were excited at 488 nm, and fluorescence was collected with a band-pass filter (500–550 nm). Fluorescence signals in figures are pseudocolored. Table 1 summarizes the color codes for various markers presented in Figures 1–6.
Table 1.
Summary of color codes for markers presented in Figures 1–6
| Figure | Panels | Red | Green | Blue | White | Yellow | Magenta |
|---|---|---|---|---|---|---|---|
| 1 | D–I | Blood vessels* | CK19 | Nuclei† and TAF‡ | |||
| J and K | Ki-67 | Nuclei and TAF | Blood vessels | Proliferating cells | |||
| 2 | A–C | Blood vessels | NG2 | Nuclei and TAF | |||
| D and F | Blood vessels | GFAP | Nuclei and TAF | ||||
| E and G | Blood vessels | GFAP | Nuclei and TAF | ||||
| 3 | A | Blood vessels | CK19 | Nuclei and TAF | |||
| A, iii | Blood vessels | CK19 | Nuclei and TAF | ||||
| B and C | Blood vessels | CK19 | Nuclei and TAF | ||||
| B, ii and C, ii | Blood vessels | CK19 | Nuclei and TAF | ||||
| 4 | D and E | Blood vessels | CK19 | Nuclei and TAF | |||
| H and I | Blood vessels | Insulin | Nuclei and TAF | ||||
| J | Blood vessels | Nuclei and TAF | Proliferating cells | Ki-67 | |||
| 5 | A | Blood vessels | GFAP | Nuclei and TAF | |||
| C and D | Blood vessels | TUJ1 | Nuclei and TAF | ||||
| 6 | A, B, and D–F | Blood vessels | NG2 | Nuclei and TAF |
Blood vessels were labeled by cardiac perfusion with lectin-Alexa Fluor 488 conjugates. †Nuclei were labeled with propidium iodide staining. ‡TAF, tissue autofluorescence.
Fig. 1.
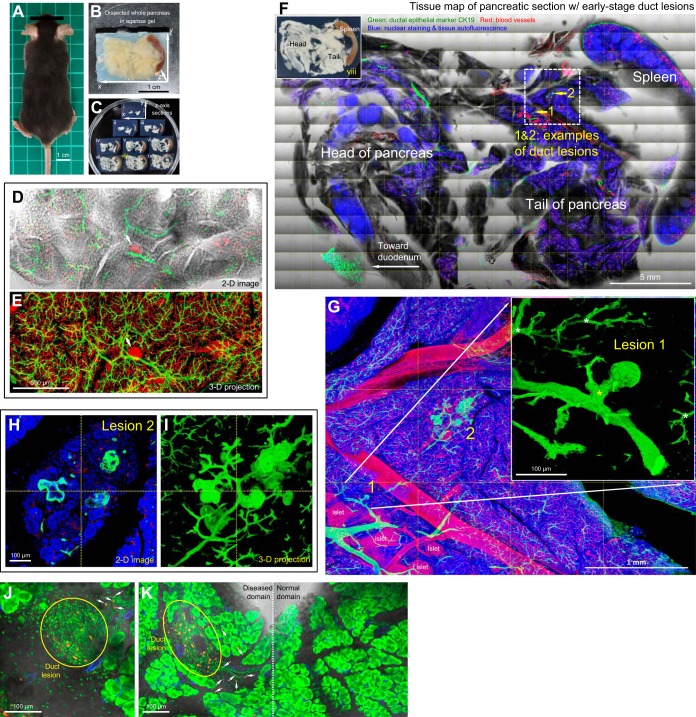
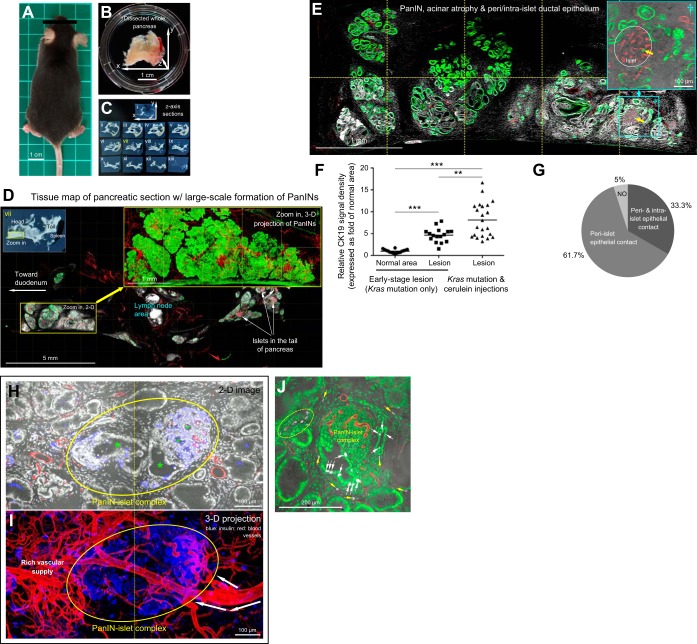
Early stage duct lesions detected by three-dimensional (3D) histology. A: Kras mutant mouse. B: pancreas. C: pancreatic sections. In C, vibratome sections of the whole pancreas were prepared along the z-axis direction shown in B. D and E: tree-like ductal network revealed by 3D histology and projection. In D, the transparent pancreas was prepared by optical clearing to allow transmitted light and in-depth microscopy. The former provides the ground-truth tissue information to verify the fluorescence signals. Projection depth in E = 300 μm. Arrow indicates an islet. Green, epithelial marker CK19; red, vascular. F: pancreatic tissue map derived from tile-scanned images. Inset: photograph of pancreatic section. Dotted box indicates an area with early stage duct lesions (enlarged in G–I). G–I: early stage duct lesions enlarged in projection. Lesions 1 and 2 are examples of epithelial buds at the duct terminals. Yellow asterisk in G inset denotes enlargement of the connecting duct to lesion 1 (in comparison with the normal ducts, white asterisks, at the same bifurcate location). Lesion 2 is presented in both 2D image and 3D projection to illustrate the epithelial buds. Green, CK19; red, vascular; blue, nuclear and tissue autofluorescence. J and K: detection of cells in proliferation in duct lesion microenvironment. Immunostaining with Ki-67 nuclear protein shows aggregation of proliferating cells (yellow) associated with the duct lesion (J and K, left) with a few scattered at the perilesional region (arrows) but not detected in the normal domain (K, right; control). Red, Ki-67; green, nuclear and tissue autofluorescence; blue, vascular.
Fig. 6.
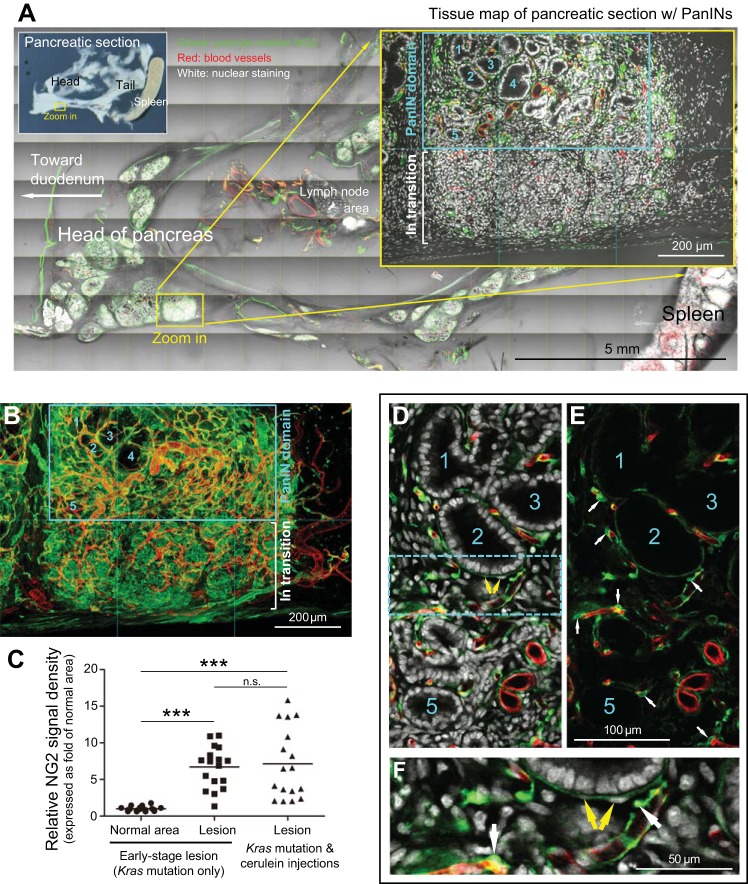
Overexpression of pericyte marker NG2 in large-scale duct lesion formation. A: tissue map of pancreatic section with PanINs. Yellow box indicates the zoom-in area. The PanIN domain and area in transition to PanIN are enlarged for examination. Numbers 1-5 denote examples of PanIN-1. Green, NG2; red, vascular; white, nuclear and tissue autofluorescence. B: projection of NG2 overexpression in large-scale duct lesion formation. The view focuses on the zoom-in area in A. NG2 overexpression is notably observed in both the PanIN domain and the area in transition. C: quantitation of NG2 overexpression in duct lesion formation. Early stage duct lesion refers to the condition in Fig. 2, A–C. The NG2 signals associated with “Lesion” refer to the signals acquired from the perilesional region. A comparable size of the area was assigned to the normal acinar domain to derive the NG2 signals in the normal area. Line indicates average of data points (***P < 0.001). Note that because pancreatic stellate cells exist in the lesion microenvironment, we did not rule out that activated stellate cells could become NG2-positive and contribute to the increase in perilesional NG2 density. D–F: NG2-positive pericytes and perilesional myofibroblast-like cells in large-scale duct lesion formation. Numbers denote the same duct lesions shown in A and B. Cyan box in D is enlarged in F. White arrows in E and F: NG2-positive perivascular pericytes. Yellow arrows in D and F: perilesional NG2-positive myofibroblast-like cells.
Image projection and analysis.
Avizo 6.2 image reconstruction software (VSG, Burlington, MA; operated with a Dell T7500 workstation), Zen software (Carl Zeiss), and LSM510 software (Carl Zeiss) were used for projection, signal segmentation, noise reduction, and analysis of confocal images. Quantitation of signal (CK19, NG2, and GFAP) density in an image stack is illustrated in (25). Briefly, to estimate density, feature extraction and image segmentation were first performed using the Label Field function in Avizo to collect voxels of lesion (or normal area) and those of CK19, NG2, or GFAP signals. Afterward, voxels with CK19, NG2, or GFAP signals in the acquired image stack were divided by those of the lesion (or normal area) × 100% to estimate signal density. In particular, in quantitation of the GFAP signals associated with PanIN (Fig. 2, E and G), we avoided the background and selected the fibrous GFAP signals that connect in space to assess the PanIN-induced gliosis. The same tissue imaging and quantitation processes were conducted on comparable normal and diseased areas to assess signal densities on the same basis.
Fig. 2.
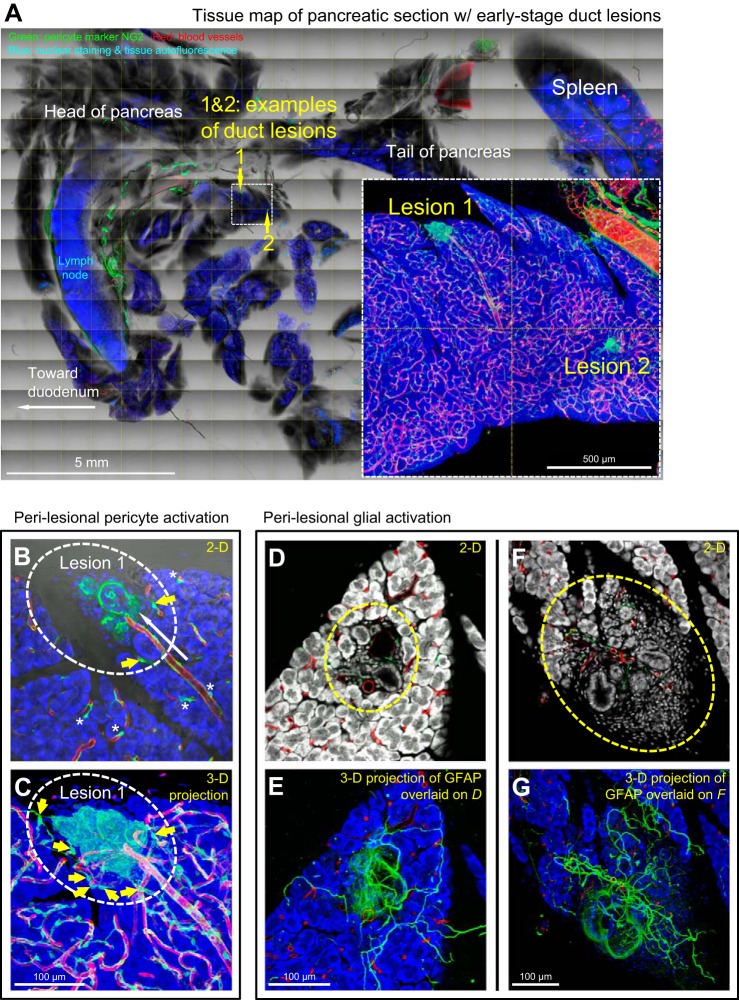
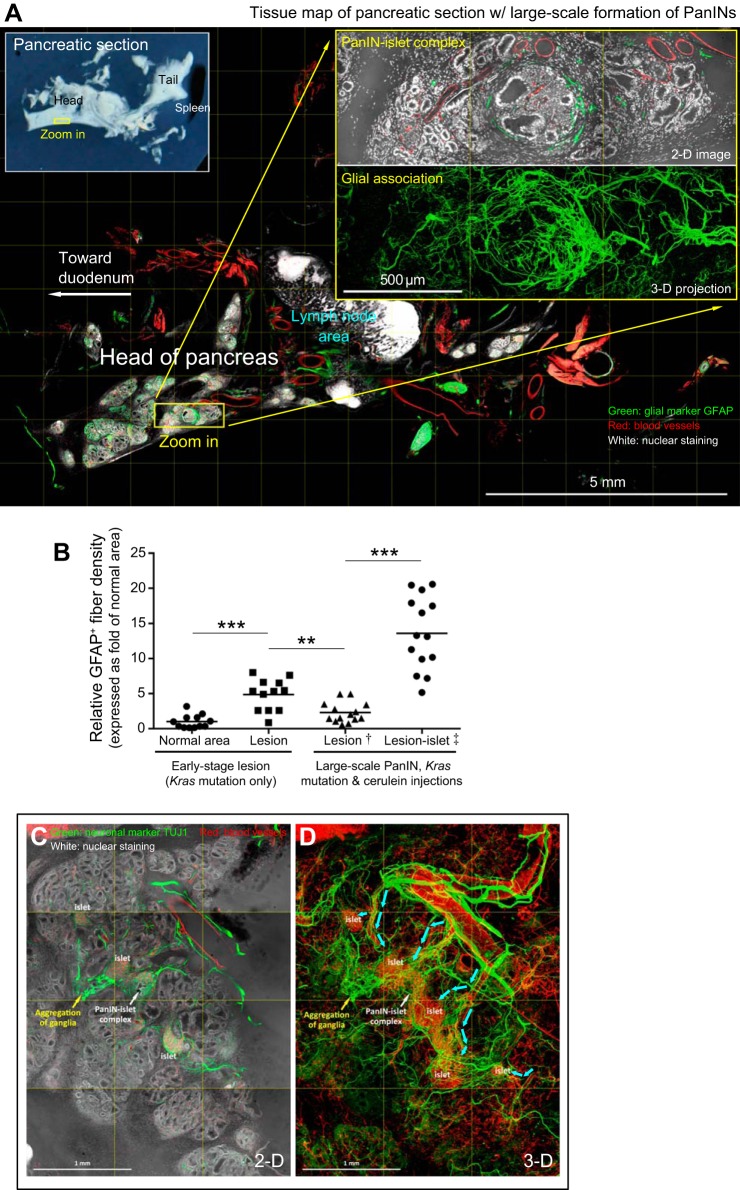
Pericyte and glial activation in early stage duct lesion formation. A–C: perilesional pericyte activation. Dotted box in A indicates the area with two examples of early stage duct lesions (enlarged in inset). Lesion 1 is furthered enlarged in B and C (oval, taken under the same view) to depict perilesional pericyte activation. Asterisks in B denote normal perivascular pericytes away from the lesion. White arrow in B indicates a perilesional microvessel. Yellow arrows in B and C indicate pericytes in contact with both microvessels and lesion. Green, pericyte marker NG2; red, vascular; blue, nuclear and tissue autofluorescence. D–G: perilesional glial activation. D and F show two examples of duct lesions at the middle and edge of the pancreatic parenchyma (oval). Overlay of 2D image and 3D projection (E and G) shows a perilesional increase in glial fibers. Green, glial fibrillary acidic protein (GFAP); red, vascular; white/blue, nuclear and tissue autofluorescence.
Statistical analysis.
Quantitative values are presented as means and distribution of data points. Statistical differences were determined with an unpaired Student's t-test. Differences between groups were considered statistically significant when P < 0.05.
RESULTS
Detection of early stage duct lesions by 3D panoramic histology.
The majority of human pancreatic ductal adenocarcinomas carry the oncogenic Kras mutation. To mimic initiation of human disease in mice, we targeted acinar cells for oncogenic Kras expression (elastase promoter-driven KrasG12D expression under the tamoxifen-inducible CreER system) to induce pancreatic duct lesion formation (20). Figure 1 shows the mouse, pancreas, and 3D morphological features of early stage duct lesions.
Survey imaging of pancreatic sections (Fig. 1C) shows that at age 14 wk (7wk after the tamoxifen injection), the majority of the pancreatic parenchyma was normal (Fig. 1, D and E), yet ∼2% of the surveyed area was detected with low-grade PanINs (Fig. 1F; PanIN-1A/B). Examples of early stage PanIN lesions are presented in Figure 1, G–I. Specifically, CK19 (epithelial marker)-labeled duct lesions deviate from the tree-like ductal network to form epithelial buds at branch terminals. Because the terminals are normally occupied by acinar cells, this result supports a prior finding that the acinar oncogenic Kras mutation induces an acinar-to-ductal metaplasia (20).
In addition to the transformation, 3D projection of the lesion microenvironment reveals an enlargement of connecting ducts to terminal buds compared with those of a normal duct at the same location (asterisks in Fig. 1G inset; 2.5 ± 0.8-fold, P < 0.001). Furthermore, cellular proliferation associated with the lesion and perilesional microenvironment is detected via Ki-67 staining (Fig. 1, J and K). These results indicate that the acinar Kras mutation influences not only acinar cells (primary target) but also the microenvironment (secondary event), including the adjacent ductal epithelium, at the early stages of duct lesion formation.
From a technical point of view, the optically cleared pancreas allows us to simultaneously use transmitted light and fluorescence imaging to construct a tissue map of the diseased pancreas (Fig. 1, D–F). In particular, the transmitted light images are important in providing ground-truth tissue information to confirm the fluorescently labeled tissue structures and networks. Once a diseased area was confirmed, we employed high-definition 3D microscopy (Fig. 1, G–I) to visualize the duct lesion and the microenvironment in an integrated fashion. In the following sections, we apply this image approach to perform qualitative and quantitative analyses of the duct lesions and associated pericyte, glial, and islet remodeling.
Pericyte and glial activation in response to early stage duct lesion formation.
Although pericyte activation has been linked to scar formation after tissue injury (18, 23), its participation in early stage duct lesion formation has not been demonstrated. Figure 2, A–C and Supplemental Video 1 show gross views and zoom-in examinations of early stage duct lesion, revealing overexpression of the pericyte marker NG2 in the perilesional region. Specifically, at this location NG2-positive pericytes (along with cells potentially derived from pericytes) aggregate with their processes surrounding the lesion and coupling to the vascular network. In comparison, when pericytes are away from the lesion (control), they are in their normal position at the abluminal domain of blood vessels. In quantitation, we used overexpression of NG2 as the indicator to assess pericyte activation. The result shows a 6.7 ± 2.8-fold (P < 0.001) increase in NG2 signals associated with the lesion against that of the normal area.
Regarding glial activation, Figure 2, D–G shows early stage duct lesions with perilesional association of the glial fibrillary acidic protein (GFAP)-labeled glial network. In a 3D presentation (Fig. 2, E and G, and Supplemental Video 1, second half) the GFAP signals connect in space, showing perilesional gliosis and an increase in GFAP staining background around the lesion [the latter is likely caused by activation of stellate cells (4, 30)]. In quantitation, we use the increase in GFAP-positive fiber density as an indicator to assess glial activation. The result shows a 4.9 ± 2.2-fold (P < 0.001) increase in the density associated with the lesion against that of the normal area.
Epithelial overgrowth in cerulein-induced chronic pancreatitis.
Next we used repetitive cerulein (cholecystokinin analog) injections to B6 mice to cause pancreatic self-digestion and chronic pancreatitis. This process led to an injury-induced formation of ductal lesions. Figure 3 shows the survey and 3D examination of the duct lesions with epithelial overgrowth caused by experimental pancreatitis.
Fig. 3.
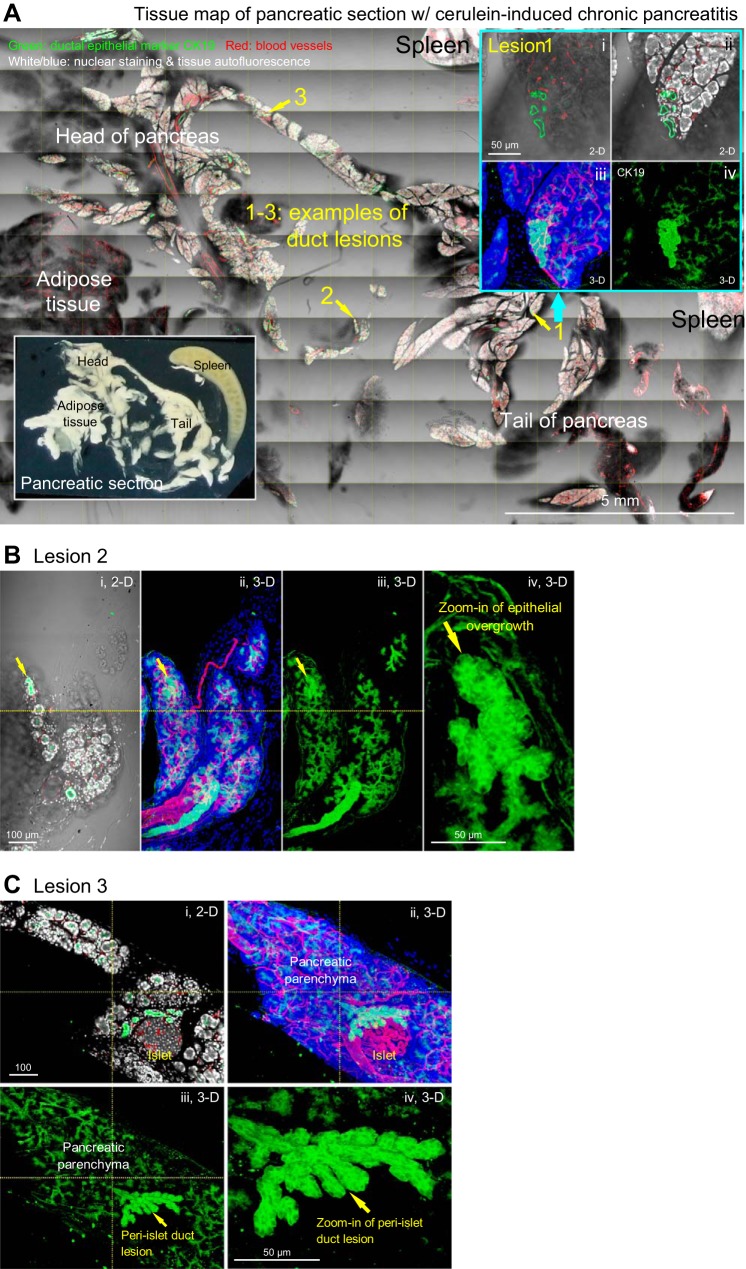
Epithelial overgrowth in cerulein-induced chronic pancreatitis. A: tissue map of mouse pancreas with cerulein-induced chronic pancreatitis. Numbers 1-3 indicate locations of duct lesions. Lesion 1 is enlarged at upper right to reveal the epithelial overgrowth at the edge of parenchyma. Green, epithelial marker CK19; red, vascular; white/blue, nuclear and tissue autofluorescence. Inset, lower left: photograph of pancreatic section. B and C: zoom-in examination of lesions 2 and 3 in A. Epithelial overgrowth is presented in both 2D image and 3D projection. B: duct lesion at the edge of parenchyma (arrow). C: duct lesion at the peri-islet region (arrow).
Two features of epithelial overgrowth were found in this animal model. First, the overgrowth (detected by CK19 staining) occurs primarily at the edge of the pancreatic lobule (Fig. 3, A and B) or at the peri-islet region (Fig. 3C), suggesting that the overgrowth is associated with pancreatic exocrine and endocrine regeneration after injury. Second, unlike lesions induced by the acinar Kras mutation, epithelial overgrowth is not associated with excessive perilesional stroma (Fig. 3 vs. Fig. 1), nor is pericyte or glial activation associated with the lesion. The result underlines the intrinsic difference of the two animal models (Kras mutation vs. cerulein-induced self-digestion) in affecting the epithelial microenvironment in the pancreas.
Remodeling of pancreatic microenvironment in large-scale duct lesion formation.
In the third animal model, we used repetitive cerulein injections to Kras-mutant mice to induce large-scale development of PanIN lesions. Figure 4, A–E shows the mouse, pancreas, and remodeled pancreatic microenvironment in this advanced stage of duct lesion formation. In particular, 3D and zoom-in examination of duct lesions show that the tree-like ductal structure is replaced by an aggregation of PanINs (PanIN-1/2; Fig. 4, D and E). In quantitation, Figure 4F summarizes increases in expression of the epithelial marker CK19, associated with the formation of duct lesions, which are 4.7 ± 1.6-fold and 8.2 ± 3.9-fold greater at early (Fig. 1, G–I) and advanced (Fig. 4, D and E) stages, respectively, compared with that of normal pancreatic area (Fig. 1, D and E) in Kras-mutant mice.
Fig. 4.
Remodeling of pancreatic microenvironment in large-scale duct lesion formation. A: Kras mutant mouse with large-scale development of PanINs. B: diseased pancreas. C: pancreatic sections. D: tissue map of pancreatic intraepithelial neoplasias (PanINs). Yellow box indicates the zoom-in area. Projection of the zoom-in area shows the aggregation of PanIN-1/2 in clusters in space. Green, epithelial marker CK19; red, vascular; white, nuclear and tissue autofluorescence. E: zoom-in examination of PanINs, acinar atrophy, and peri-islet/intraislet ductal epithelium. Islet at lower right is enlarged to show the peri- and intraislet epithelium (green, arrow). F: increase in epithelial marker CK19 expression in duct lesion formation. Early stage duct lesion refers to the condition in Fig. 1, G–I. The CK19 signals associated with “lesion” refer to the signals derived from the epithelial buds. A comparable size of the area was assigned to the normal domain to derive the CK19 signals in the “normal area.” Line indicates the average of data points (**P < 0.01, ***P < 0.001). G: percentage of islets with peri-islet and/or intraislet epithelial contact in large-scale duct lesion formation, n = 60 islets (derived from four mice). H and I: PanIN-islet complex revealed by paired insulin and nuclear staining. Blue, insulin; white, nuclear and tissue autofluorescence; red, vascular. Oval: PanIN-islet complex. Asterisks in H: lumens of peri- and intra-islet duct lesions. White arrows in I: feeding arteriole of the PanIN-islet complex. J: PanIN-islet complex and proliferating epithelial and stromal cells. Ki-67 staining (magenta) was used to identify proliferating cells (white; overlap with nuclear signals, green). White arrows denote Ki-67-positive epithelial cells. Ki-67-positive stromal cells appear at the perilesional region (oval and yellow arrows). Red, vascular staining.
In addition to exocrine remodeling, surprisingly, we also detected the endocrine islets being affected by large-scale duct lesion formation. This unexpected finding is featured with the in-growth of ductal epithelium to the islets, forming the PanIN-islet complex (inset in Fig. 4E). We estimate that 95% of islets are in direct contact with duct lesions, which occurs at the peri-islet (61.7%) or both the peri- and intraislet regions (33.3%) (Fig. 4G). This unique PanIN-islet complex is confirmed by paired insulin and nuclear staining (Fig. 4, H and I) and is found associated with proliferating epithelial and stromal cells (Fig. 4J and Supplemental Video 2), implicating an atypical relationship among the endocrine islet, the diseased epithelium, and the stroma in large-scale duct lesion formation.
Rich neurovascular tissues are associated with the PanIN-islet complex.
The endocrine islet receives substantial neurovascular supplies to regulate hormone secretion. This arrangement allows islet-associated PanINs to be surrounded with a higher density of neurovascular tissues compared with those away from islets. Figure 5A reveals a prominent glial association with the PanIN-islet complex and surrounding duct lesions. Supplemental Video 3 presents an in-depth recording of the association. In quantitation, Figure 5B summarizes the increase in GFAP-positive glial fiber density associated with early stage duct lesion (a 4.9-fold increase compared with normal area), and separately, with the PanIN-islet complex (a 5.9-fold increase compared with PanIN lesions away from islets).
Fig. 5.
PanIN-islet complex and its rich neurovascular association. A: PanIN-islet complex in large-scale duct lesion formation. Condensed glial fibers can be seen associated with the PanIN-islet complex and the surrounding duct lesions (shown in 2D image and 3D projection). Yellow box indicates the zoom-in area. Green, glial marker GFAP; red, vascular; white, nuclear and tissue autofluorescence. B: increase in GFAP-positive glial fiber density associated with early stage duct lesion and PanIN-islet complex. †Lesions away from the islet (>1 mm); ‡PanIN-islet complex. Line indicates average of data points (**P < 0.01, ***P < 0.001). C and D: PanIN-islet complex and neurovascular aggregation in large-scale duct lesion formation. White and yellow arrows indicate the PanIN-islet complex and surrounding ganglial aggregation. Cyan arrows indicate feeding arterioles of the PanIN-islet complex. Neuronal network and ganglia are revealed by TUJ1 staining (green).
Interestingly, when we compare the glial fiber density of early stage lesions (Kras mutation only) with that of the advanced PanIN lesions away from islets (Kras mutation with additional cerulein pancreatitis; dagger in Fig. 5B), the latter shows a 53% decrease in perilesional glial association. This decrease reflects the difference in the two duct lesion models. The former uses Kras mutation to induce lesion formation; the latter applies additional cerulein injections to cause pancreatic self-digestion, which appears to have changed the perilesional microenvironment previously established by the Kras mutation.
Importantly, in addition to the glial network, nerves (labeled by neuronal marker TUJ1 staining) and blood vessels are also notably observed as being associated with PanIN-islet complexes, making them landmarks in an atrophic lobe (Fig. 5, C and D). Overall, identification of the PanIN-islet complex and its rich neurovascular association underlines the heterogeneity of the duct lesion microenvironment and suggests the potential influence of duct lesions on islets through paracrine and neurovascular contacts/connections.
Overexpression of the pericyte marker NG2 in large-scale duct lesion formation.
Stromal proliferation and stromal-epithelial interactions are important features in the development of PanINs. In large-scale duct lesion formation, expression of the pericyte marker NG2 is prominent in stroma and in adjacent pancreatic areas in transition to the lesion (Fig. 6, A and B). The level of perilesional NG2 expression in this condition is comparable to that of early stage duct lesions (Fig. 6C), with the former showing a 7.1-fold increase compared with NG2 signals in normal pancreatic areas. Importantly, when we zoomed into the stromal microenvironment, Figure 6, D–F reveals that not only pericytes but also perilesional myofibroblast-like cells are NG2-positive. NG2 overexpression and the simultaneous presence of NG2-positive pericytes and myofibroblast-like cells indicate pericyte activation and suggests a potential pericyte-to-myofibroblast transition in the stroma, which appears in renal injury and fibrosis (23, 12).
DISCUSSION
The acinar Kras mutation has been used in animal models to study the origin and progression of pancreatic ductal adenocarcinoma (1, 10, 20). However, in the process, early events in ductal and neurovascular remodeling have not been systematically demonstrated because of a lack of imaging tools to globally examine the lesion microenvironment. In this research, we first combined 3D duct lesion histology with tile scanning to identify early stage duct lesions and revealed perilesional pericyte and glial activation. Next, the same imaging strategy was applied to examine the large-scale development of PanINs, which was induced by cerulein injections to Kras mutant mice. On the basis of 3D image data, we identified 1) the aggregation of PanINs in clusters in space; 2) overexpression of the pericyte marker NG2 in the PanIN microenvironment; and 3) epithelial in-growth to islets, forming PanIN-islet complexes. These results highlight the epithelial remodeling and neurovascular and endocrine links to the progression of PanINs.
Perilesional increases in NG2 expression and GFAP-positive fibers confirm the reactivity of pericytes and glial cells in response to early stage duct lesion formation. Because activation of the two cell types was also identified in early insulitis (40), these results suggest the sensitivity of pericytes and glial cells in detecting subtle changes in the pancreatic microenvironment and their potential use as early stage markers for lesion detection. Also, because pericytes and glial cells are known to release angiogenic and neurotrophic factors for neurovascular recruitment (16, 21), we suspect that their activation is linked to structural remodeling (i.e., formation of the epithelial bud and stroma; Fig. 1) to support ongoing cellular transformation. Notably, in histogenesis and tumorigenesis, the surface molecule NG2—a chondroitin sulfate proteoglycan—is found on immature cells, in which the NG2 signaling pathway has been implicated in cellular proliferation and motility (26, 29, 37). In this research, the discovery of PanIN-associated NG2 overexpression (Fig. 6) is in accord with the staining data of Ki-67-positive proliferating cells (Fig. 1, J and K, and Fig. 4J) to indicate that the pancreatic microenvironment is in transition to a state of active proliferation, deviating from the quiescent, differentiated status.
The PanIN-islet complex highlights morphological evidence of endocrine islets that are influenced by diseased epithelium at an advanced stage of duct lesion formation (Figs. 4 and 5). The development of peri- and intraislet ducts is similar to that of ductal in-growth to the islets in young db/db mice, in which hyperphagic obesity leads to insulin resistance and diabetes (5, 7). Clinically, the progression of pancreatic duct lesions and diabetes held a bidirectional relationship: roughly one-half of patients with pancreatic cancer have diabetes, and patients with new-onset diabetes have an increased risk of being diagnosed with pancreatic cancer (32, 35, 36). In particular, the paraneoplastic diabetes is similar to type 2 diabetes in insulin resistance, hyperinsulinemia, and β-cell dysfunction. Thus our observation of similar peri- and intraislet ducts in animal models of PanIN and type 2 diabetes (db/db mice) indicates that endocrine islets in both conditions are under stress with abnormal paracrine influences from the epithelium to the islets. In the reverse direction, because insulin has been known to promote cellular proliferation, higher intrapancreatic insulin levels around the islet are likely to provide a growth advantage to islet-associated PanINs (Fig. 4J).
Technical advances in preparation of transparent pancreata enabled the development of 3D duct lesion histology [Fig. 1 and (7, 8, 15)]. Dispersed ductal and neurovascular networks were revealed by deep-tissue microscopy, which allowed tissue information to be visualized within a 3D space continuum. Spatial information is important in delineating novel details of network remodeling in the diseased pancreas, which otherwise cannot be visualized with standard microtome-based histology. In addition, we adopted an imaging strategy of using both tile-scanning and 3D microscopy to identify and characterize duct lesions. This approach provides global and continuous tissue information to avoid uncertainties in depicting the intricate microenvironment of PanINs.
In summary, using the experimental acinar Kras mutation, we have identified PanIN-associated pericyte, glial, and islet remodeling via 3D duct lesion histology. Before this research, development of PanIN lesions was examined only locally, and without a clear picture of associated tissue remodeling in the pancreatic microenvironment. Our demonstration of perilesional pericyte and glial activation and discovery of the PanIN-islet complex lays the technical and scientific foundations to study the neurovascular and endocrine links to human PanIN progression.
GRANTS
This work was supported in part by grants from Taiwan National Health Research Institutes (NHRI-EX103-10134BI), Ministry of Science and Technology (MOST 104-2321-B-001-028 and 105-0210-01-13-01), and Academia Sinica (intramural) to C.-N. Shen; NHRI-EX105-10524EI and MOST 104-2314-B-007-002-MY2 to S.-C. Tang.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
P.-Y.L., S.-J.P., C.-N.S., P.J.P., and S.-C.T. conception and design of research; P.-Y.L. and S.-J.P. performed experiments; P.-Y.L., S.-J.P., C.-N.S., P.J.P., and S.-C.T. analyzed data; P.-Y.L., S.-J.P., C.-N.S., P.J.P., and S.-C.T. interpreted results of experiments; P.-Y.L. and S.-C.T. prepared figures; C.-N.S., P.J.P., and S.-C.T. drafted manuscript; P.-Y.L., S.-J.P., C.-N.S., P.J.P., and S.-C.T. edited and revised manuscript; P.-Y.L., S.-J.P., C.-N.S., P.J.P., and S.-C.T. approved final version of manuscript.
Supplemental Data
Supplemental Video 1 (related to Figure 2): In-depth recording of peri-lesional pericyte and glial activation in early-stage duct lesion formation. First half: peri-lesional pericyte activation. Second half: peri-lesional glial activation - .mp4 (7 MB)
Supplemental Video 2 (related to Figure 4): In-depth recording of proliferating epithelial and stromal cells associated with PanIN-islet complex. Red: vascular staining. Green: nuclear staining. Magenta/white: ki67+ nuclei of proliferating cells - .mp4 (4 MB)
Supplemental Video 3 (related to Figure 5A): In-depth recording of PanIN-islet complex and the glial network association. Glial fibers are prominently seen associated with the PanIN-islet complex (middle) and the surrounding duct lesions. Green: glial marker GFAP staining. Red: vascular staining. White: nuclear staining and tissue autofluorescence - .mp4 (13 MB)
ACKNOWLEDGMENTS
We thank the confocal imaging core and brain research center at National Tsing Hua University for technical support in 3D microscopy and postrecording image processing.
For correspondence regarding mouse models of PanIN: C. N. Shen (e-mail: cnshen@gate.sinica.edu.tw). For correspondence regarding 3D pancreatic histology: S. C. Tang (e-mail: sctang@life.nthu.edu.tw).
REFERENCES
- 1.Aguirre AJ, Bardeesy N, Sinha M, Lopez L, Tuveson DA, Horner J, Redston MS, DePinho RA. Activated Kras and Ink4a/Arf deficiency cooperate to produce metastatic pancreatic ductal adenocarcinoma. Genes Dev : 3112–3126, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahren B. Autonomic regulation of islet hormone secretion—implications for health and disease. Diabetologia : 393–410, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Ahren B. Islet nerves in focus—defining their neurobiological and clinical role. Diabetologia : 3152–3154, 2012. [DOI] [PubMed] [Google Scholar]
- 4.Apte MV, Yang L, Phillips PA, Xu Z, Kaplan W, Cowley M, Pirola RC, Wilson JS. Extracellular matrix composition significantly influences pancreatic stellate cell gene expression pattern: role of transgelin in PSC function. Am J Physiol Gastrointest Liver Physiol : G408–G417, 2013. [DOI] [PubMed] [Google Scholar]
- 5.Boquist L, Hellman B, Lernmark A, Täljedal IB. Influence of the mutation “diabetes” on insulin release and islet morphology in mice of different genetic backgrounds. J Cell Biol : 77–89, 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen YT, Chang FC, Wu CF, Chou YH, Hsu HL, Chiang WC, Shen J, Chen YM, Wu KD, Tsai TJ, Duffield JS, Lin SL. Platelet-derived growth factor receptor signaling activates pericyte-myofibroblast transition in obstructive and post-ischemic kidney fibrosis. Kidney Int : 1170–1181, 2011. [DOI] [PubMed] [Google Scholar]
- 7.Chien HJ, Peng SJ, Hua TE, Kuo CH, Juang JH, Tang SC. 3-D imaging of islets in obesity: formation of the islet-duct complex and neurovascular remodeling in young hyperphagic mice. Int J Obes (Lond) : 685–697, 2016. [DOI] [PubMed] [Google Scholar]
- 8.Chiu YC, Hua TE, Fu YY, Pasricha PJ, Tang SC. 3-D imaging and illustration of the perfusive mouse islet sympathetic innervation and its remodelling in injury. Diabetologia : 3252–3261, 2012. [DOI] [PubMed] [Google Scholar]
- 9.Demir IE, Ceyhan GO, Rauch U, Altintas B, Klotz M, Müller MW, Büchler MW, Friess H, Schäfer KH. The microenvironment in chronic pancreatitis and pancreatic cancer induces neuronal plasticity. Neurogastroenterol Motil : 480–490, 2010. [DOI] [PubMed] [Google Scholar]
- 10.di Magliano MP, Logsdon CD. Roles for KRAS in pancreatic tumor development and progression. Gastroenterology : 1220–1229, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donev SR. Ultrastructural evidence for the presence of a glial sheath investing the islets of Langerhans in the pancreas of mammals. Cell Tissue Res : 343–348, 1984. [DOI] [PubMed] [Google Scholar]
- 12.Duffield JS. Cellular and molecular mechanisms in kidney fibrosis. J Clin Invest : 2299–2306, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fawcett JW, Asher RA. The glial scar and central nervous system repair. Brain Res Bull : 377–391, 1999. [DOI] [PubMed] [Google Scholar]
- 14.Fu YY, Lin CW, Enikolopov G, Sibley E, Chiang AS, Tang SC. Microtome-free 3-dimensional confocal imaging method for visualization of mouse intestine with subcellular-level resolution. Gastroenterology : 453–465, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fu YY, Lu CH, Lin CW, Juang JH, Enikolopov G, Sibley E, Chiang AS, Tang SC. Three-dimensional optical method for integrated visualization of mouse islet microstructure and vascular network with subcellular-level resolution. J Biomed Opt : 046018, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gerhardt H, Betsholtz C. Endothelial-pericyte interactions in angiogenesis. Cell Tissue Res : 15–23, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Gittes GK. Developmental biology of the pancreas: a comprehensive review. Dev Biol : 4–35, 2009. [DOI] [PubMed] [Google Scholar]
- 18.Goritz C, Dias DO, Tomilin N, Barbacid M, Shupliakov O, Frisen J. A pericyte origin of spinal cord scar tissue. Science : 238–242, 2011. [DOI] [PubMed] [Google Scholar]
- 19.Guerra C, Schuhmacher AJ, Cañamero M, Grippo PJ, Verdaguer L, Pérez-Gallego L, Dubus P, Sandgren EP, Barbacid M. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell : 291–302, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Habbe N, Shi G, Meguid RA, Fendrich V, Esni F, Chen H, Feldmann G, Stoffers DA, Konieczny SF, Leach SD, Maitra A. Spontaneous induction of murine pancreatic intraepithelial neoplasia (mPanIN) by acinar cell targeting of oncogenic Kras in adult mice. Proc Natl Acad Sci USA : 18913–18918, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henderson CE, Phillips HS, Pollock RA, Davies AM, Lemeulle C, Armanini M, Simmons L, Moffet B, Vandlen RA, Simpson LC corrected to Simmons L, Koliatsos VE, Rosenthal A. GDNF: a potent survival factor for motoneurons present in peripheral nerve and muscle. Science : 1062–1064, 1994. [DOI] [PubMed] [Google Scholar]
- 22.Hruban RH, Adsay NV, Albores-Saavedra J, Compton C, Garrett ES, Goodman SN, Kern SE, Klimstra DS, Kloppel G, Longnecker DS, Luttges J, Offerhaus GJ. Pancreatic intraepithelial neoplasia: a new nomenclature and classification system for pancreatic duct lesions. Am J Surg Pathol : 579–586, 2001. [DOI] [PubMed] [Google Scholar]
- 23.Humphreys BD, Lin SL, Kobayashi A, Hudson TE, Nowlin BT, Bonventre JV, Valerius MT, McMahon AP, Duffield JS. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol : 85–97, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Juang JH, Kuo CH, Peng SJ, Tang SC. 3-D imaging reveals participation of donor islet Schwann cells and pericytes in islet transplantation and graft neurovascular regeneration. EBioMedicine : 109–119, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Juang JH, Peng SJ, Kuo CH, Tang SC. Three-dimensional islet graft histology: panoramic imaging of neural plasticity in sympathetic reinnervation of transplanted islets under the kidney capsule. Am J Physiol Endocrinol Metab : E559–E570, 2014. [DOI] [PubMed] [Google Scholar]
- 26.Levine JM, Nishiyama A. The NG2 chondroitin sulfate proteoglycan: a multifunctional proteoglycan associated with immature cells. Perspect Dev Neurobiol : 245–259, 1996. [PubMed] [Google Scholar]
- 27.Lindahl P, Johansson BR, Leveen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science : 242–245, 1997. [DOI] [PubMed] [Google Scholar]
- 28.Lowenfels AB, Maisonneuve P, Cavallini G, Ammann RW, Lankisch PG, Andersen JR, Dimagno EP, Andrén-Sandberg A, Domellöf L. Pancreatitis and the risk of pancreatic cancer. International Pancreatitis Study Group. N Engl J Med : 1433–1437, 1993. [DOI] [PubMed] [Google Scholar]
- 29.Makagiansar IT, Williams S, Mustelin T, Stallcup WB. Differential phosphorylation of NG2 proteoglycan by ERK and PKCα helps balance cell proliferation and migration. J Cell Biol : 155–165, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moir JA, Mann J, White SA. The role of pancreatic stellate cells in pancreatic cancer. Surg Oncol : 232–238, 2015. [DOI] [PubMed] [Google Scholar]
- 31.Mwangi S, Anitha M, Mallikarjun C, Ding X, Hara M, Parsadanian A, Larsen CP, Thule P, Sitaraman SV, Anania F, Srinivasan S. Glial cell line-derived neurotrophic factor increases beta-cell mass and improves glucose tolerance. Gastroenterology : 727–737, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pannala R, Basu A, Petersen GM, Chari ST. New-onset diabetes: a potential clue to the early diagnosis of pancreatic cancer. Lancet Oncol : 88–95, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ravnic DJ, Jiang X, Wolloscheck T, Pratt JP, Huss H, Mentzer SJ, Konerding MA. Vessel painting of the microcirculation using fluorescent lipophilic tracers. Microvasc Res : 90–96, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Richards OC, Raines SM, Attie AD. The role of blood vessels, endothelial cells, and vascular pericytes in insulin secretion and peripheral insulin action. Endocr Rev : 343–363, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sah RP, Nagpal SJ, Mukhopadhyay D, Chari ST. New insights into pancreatic cancer-induced paraneoplastic diabetes. Nat Rev Gastroenterol Hepatol : 423–433, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salvatore T, Marfella R, Rizzo MR, Sasso FC. Pancreatic cancer and diabetes: a two-way relationship in the perspective of diabetologist. Int J Surg , Suppl 1: S72–S77, 2015. [DOI] [PubMed] [Google Scholar]
- 37.Stallcup WB, Huang FJ. A role for the NG2 proteoglycan in glioma progression. Cell Adh Migr : 192–201, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strobel O, Dor Y, Alsina J, Stirman A, Lauwers G, Trainor A, Castillo CF, Warshaw AL, Thayer SP. In vivo lineage tracing defines the role of acinar-to-ductal transdifferentiation in inflammatory ductal metaplasia. Gastroenterology : 1999–2009, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sunami E, Kanazawa H, Hashizume H, Takeda M, Hatakeyama K, Ushiki T. Morphological characteristics of Schwann cells in the islets of Langerhans of the murine pancreas. Arch Histol Cytol : 191–201, 2001. [DOI] [PubMed] [Google Scholar]
- 40.Tang SC, Chiu YC, Hsu CT, Peng SJ, Fu YY. Plasticity of Schwann cells and pericytes in response to islet injury in mice. Diabetologia : 2424–2434, 2013. [DOI] [PubMed] [Google Scholar]
- 41.Teitelman G, Guz Y, Ivkovic S, Ehrlich M. Islet injury induces neurotrophin expression in pancreatic cells and reactive gliosis of peri-islet Schwann cells. J Neurobiol : 304–318, 1998. [DOI] [PubMed] [Google Scholar]
- 42.Tuveson DA, Shaw AT, Willis NA, Silver DP, Jackson EL, Chang S, Mercer KL, Grochow R, Hock H, Crowley D, Hingorani SR, Zaks T, King C, Jacobetz MA, Wang L, Bronson RT, Orkin SH, DePinho RA, Jacks T. Endogenous oncogenic K-rasG12D stimulates proliferation and widespread neoplastic and developmental defects. Cancer Cell : 375–387, 2004. [DOI] [PubMed] [Google Scholar]
- 43.von Tell D, Armulik A, Betsholtz C. Pericytes and vascular stability. Exp Cell Res : 623–629, 2006. [DOI] [PubMed] [Google Scholar]
- 44.Wu SY, Hsieh CC, Wu RR, Susanto J, Liu TT, Shen CR, Chen Y, Su CC, Chang FP, Chang HM, Tosh D, Shen CN. Differentiation of pancreatic acinar cells to hepatocytes requires an intermediate cell type. Gastroenterology : 2519–2530, 2010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Video 1 (related to Figure 2): In-depth recording of peri-lesional pericyte and glial activation in early-stage duct lesion formation. First half: peri-lesional pericyte activation. Second half: peri-lesional glial activation - .mp4 (7 MB)
Supplemental Video 2 (related to Figure 4): In-depth recording of proliferating epithelial and stromal cells associated with PanIN-islet complex. Red: vascular staining. Green: nuclear staining. Magenta/white: ki67+ nuclei of proliferating cells - .mp4 (4 MB)
Supplemental Video 3 (related to Figure 5A): In-depth recording of PanIN-islet complex and the glial network association. Glial fibers are prominently seen associated with the PanIN-islet complex (middle) and the surrounding duct lesions. Green: glial marker GFAP staining. Red: vascular staining. White: nuclear staining and tissue autofluorescence - .mp4 (13 MB)