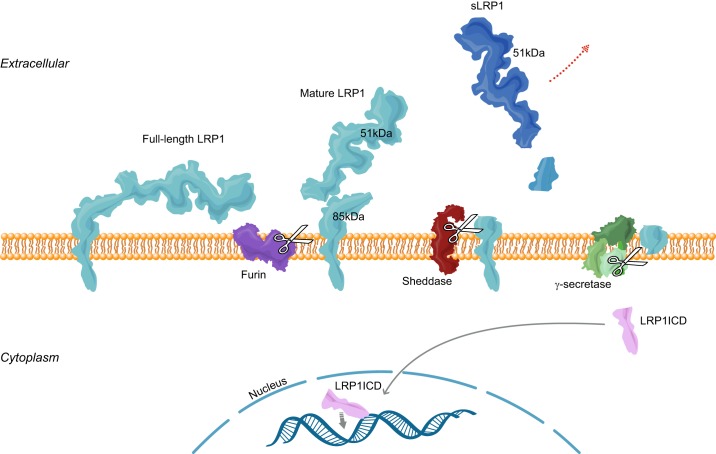
FIGURE 2.
LRP1 proteolytic processing. Full-length LRP1 is cleaved by furin into 515 and 85 kDa subunits, which associate with each other noncovalently as “mature LRP1.” The 85-kDa subunit can be processed by sheddases to free the larger subunit (soluble LRP1, or sLRP1; dark blue) from the membrane. The remaining transmembrane subunit may be further cleaved by γ-secretase to release the LRP1 intracellular domain (LRP1ICD; pink), which can translocate to the nucleus to regulate gene transcription.