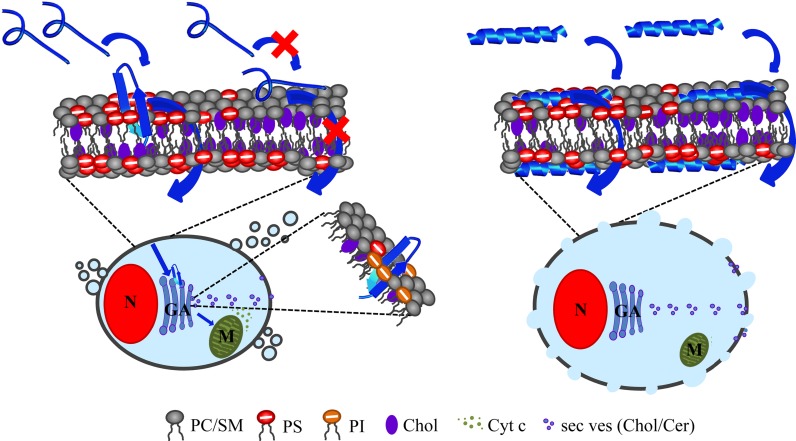
Fig 13. Predicted modes of action of R-DIM-P-LF11-322 and DIM-LF11-318 on cancer cells.
Cancer cells specifically expose the negatively charged lipid phosphatidylserine (PS, red) in the outer leaflet of the plasma membrane [7,15] offering a target for the cationic amphipathic peptides. Cholesterol (Chol, purple) is enriched in so called rafts together with phosphatidylcholine and sphingomyelin (PC and SM, gray) [65]. Both peptides cause severe changes in lipid distribution, leading to defect borderlines and via primary and secondary effects to different ways of cell death. Left: R-DIM-P-LF11-322 (blue) forms its membrane active structure (β-sheet) only in presence of PS [28,30], which however can be partially hindered by the presence of cholesterol. At PS enriched sites, though, R-DIM-P-LF11-322 with its active structure can successfully enter the cell and reach an intracellular target, presumably the Golgi apparatus (GA) harboring negatively charged lipids as PS or phosphatidylinositol (PI, orange) leading to lipid trafficking, domain formation in the plasma membrane and mitochondrial swelling. Apoptosis occurs via different ways, e.g., by inducing loss of membrane potential of mitochondria (M) which leads to cytochrome c (Cyt c) efflux and transport of secretory vesicles (sec ves) carrying Chol and ceramide (Cer). Right: DIM-LF11-318 (blue) forms its membrane active structure (α-helix) in presence of both, PS and PC, independently of cholesterol. DIM-LF11-318 is thought to harm the cell rapidly and directly at the plasma membrane leading to necrosis indicated by membrane lysis and release of cell debris. Intracellularly, strong stress is induced shown by lipid trafficking to and changes in domains of the plasma membrane. Modified from Riedl et al. [30].