Abstract
Development of portal hypertension (PHT) is a central prognostic factor in patients with cirrhosis. Circulating microparticles (MPs) are released by hepatocytes in a caspase dependent manner, are increased in circulation of patients with cirrhosis and contribute to PHT via induction of impaired vasoconstrictor responses. Here we tested the hypothesis that emricasan, a pan-caspase inhibitor, ameliorates PHT and reduction in release of MPs. We used a short-term and long-term protocol following common bile-duct ligation (BDL) in C57BL/6 mice (10 days and 20 days, respectively). Mice were treated daily via intraperitoneal injection with 10 mg/kg/day of emricasan or placebo. Circulating MP levels were analyzed using flow cytometry and function via ex-vivo angiogenesis assays. In contrast, to BDL-placebo group, nearly all BDL-emricasan treated mice survived after long-term BDL. Assessment of portal pressure showed a significant increase in BDL-placebo mice compared to sham-placebo mice. In contrast, BDL-emricasan mice had significantly lower levels of portal pressure compared to BDL-placebo mice. Although emricasan treatment resulted in a decrease in fibrosis the changes did not reach statistical significance suggesting that the effects on PHT are at least in part independent of the anti-fibrotic effects of the drug. Following short-term BDL, hepatocellular cell death as well as liver fibrosis had improved and circulating MPs were significantly reduced in BDL-emricasan mice compared to BDL-placebo. Circulating MPs from BDL-placebo mice induced endothelial cell activation and this was significantly reduced in MPs from BDL-emricasan mice. Our results indicate that emricasan treatment improves survival and PHT in a murine model of long-term BDL. Emricasan is a promising agent for the treatment of PHT.
Keywords: portal hypertension, cirrhosis, pan-caspase inhibitor, extracellular vesicles
Introduction
The development of portal hypertension (PHT) is a central consequence of advanced liver disease and cirrhosis and a key contributor to the leading complications of cirrhosis including the formation of esophageal and gastric varices responsible for variceal bleeding, associated with a high mortality rate, as well as other severe complications such as portosystemic encephalopathy and sepsis [1, 2]. The mechanisms resulting in PHT in cirrhosis are thought to be mainly a result of increased sinusoidal resistance to flow due to hepatic fibrosis and nodule formation but also due to hemodynamic changes resulting in increased portal inflow. Extracellular vesicles, including exosomes and microparticles (MP) are an heterogeneous population of small membrane-bound structures released by cells in the extracellular environment as well as in the bloodstream [3]. MPs are effective communicators that are generated by a cell of origin or parenteral cell and can act on a number of target cells in an autocrine or paracrine pathway in the extracellular environment where they are released, as well as in an endocrine manner, acting as long-range signals. Circulating MPs containing cytokeratin-18 and expressing phosphatidylserine on the surface are released by hepatocytes in a caspase dependent manner [4, 5] and have been shown to be increased in circulation of patients with cirrhosis, and in these patients to impair vasoconstrictor responses and decrease blood pressure [6], contributing to the arterial vasodilation that may represent an important contributor to the worsening of PHT. Broad-spectrum caspase inhibition via the use of pan-caspase inhibitors is a novel therapeutic approach that has been shown to have anti-inflammatory and anti-fibrotic effects in experimental models and in patients with chronic liver disease [7–10]. In this study, we investigated the effects of emricasan (IDN-6556) a pan-caspase inhibitor on PHT as well as the potential mechanism involved in these effects by using both short-term (10-days) and long-term (20 days) BDL in mice.
Materials and Methods
Animal Studies
The use and care of the animals was reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) at the University of California, San Diego (UCSD).
Secondary biliary cirrhosis was induced by long-term common BDL in C57BL/6 mice (20 – 25 grams of body weight), which were purchased from Harlan Laboratories (CA, USA). Controls were sham-operated, the abdominal cavity opened, but no ligature placed. Mice were treated with 10 mg/kg/day of IDN-6556 or placebo (0.9% saline chloride) for 10 or 20 days via intraperitoneal (i.p.) injection (4 groups, n= 7-16 in each group). First injection was performed right after operation. Only animals that survived 5 days were included in the study because early deaths were attributed to the surgical procedure. After 20 days, portal pressure using catheter from ileocolic vein was measured in each mouse.
Liver and blood sample preparation
All mice were sacrificed at the termination of treatment under anesthesia via i.p. injection using a 21G needle and a mixture of 100 mg/kg of Ketamine and 10 mg/kg of Xylazine dissolved in a 0.9% saline solution with euthanasia carried out by carbon dioxide exposure. Whole mouse blood was collected by cardiac puncture into tubes containing anticoagulant. Liver tissue was fixed in 10% formalin for 24 h and embedded in paraffin, and the remaining liver tissue was quickly frozen in liquid nitrogen and stored at −80°C. ALT value was measured with Infinity ALT liquid stable reagents (Thermo Fisher scientific).
Liver histology and Immunostaining
Tissue sections were prepared and stained for hematoxylin and eosin. Steatosis and liver fibrosis, cholangiocyte proliferation, liver angiogenesis or liver cell death were assessed via Sirius Red staining - liver sections were incubated with Fast Green FCF (Thermo Fisher scientific) and Direct Red (Sigma-Aldrich) in saturated picric acid (Sigma-Aldrich) for 2 h at room temperature, immunohistochemistry using α-SMA (Abcam), CK19 (Developmental studies hybridoma bank), and CD31 (Abcam) antibodies, or terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assay (Millipore) according to the manufacture’s protocol. All pictures were taken by NanoZoomer 2.0HT Slide Scanning System (Hamamatsu) and quantitated on Image J software.
Microparticles
Blood was centrifuged at 1,200 g for 15 min and 12,000 g for 12 min at 22°C to obtain platelet free plasma (PFP). PFP was incubated with Annexin-V (Life Technologies) according to manufacturer’s instruction. MP count was performed using 2.5-μm Alignflow alignment beads (Thermo Fisher Scientific) as the size standards for flow cytometry, BD LSRII Flow Cytometer System, (Thermo Fisher Sciences). The data were analyzed using FlowJo software (TreeStar Inc.). MPs were purified via ultracentrifugation at 20,000 g for 30 min at 4°C. For internalization, MPs were stained with PKH26 red fluorescent cell linker kit (Sigma) according to manufacturer’s protocol and purified via ultracentrifugation to remove free dye. For detection of VEGF in MPs, MPs were lysed with RIPA buffer and resolved by Criterion™ TGX Gel (Bio-Rad). Proteins were transferred to 0.2 μm nitrocellulose membrane (Bio-Rad) and blots were then hybridized overnight using antibody VEGF (Abcam) flowing by secondary antibody. Proteins were visualized by SuperSignal West chemiluminescence substrate (Pierce biotechnology) and band intensity was analyzed using Image Lab (Bio-Rad).
Cell cultures and Tube formation
Human umbilical vein endothelial cells (HUVECs) were maintained in EGM-2 growth medium (Lonza) supplemented with several angiogenic and growth factors (SingleQuots, Lonza) according to manufacturer’s protocol. Cells were cultured at 37°C in a 5% CO2. For tube formation, HUVECs were incubated with growth factor–free medium (EBM-2, Lonza). HUVECs were seeded onto a coated 24-well plate with Matrigel (200 μl/cm2; BD) using the thick gel method and treated with VEGF (50 ng/ml; PeproTech), or MPs in EBM-2 basal medium for 3 h at 37°C. HUVECs were stained via calcein-AM (Thermo Fisher scientific) and tube formation was investigated by capturing images at ×4 magnification. Tube formation image analysis was assessed with Wimtube software (Wimasis), and the values of total tube length (pixel) were used for statistical and data analysis.
Statistical analyses
All data are expressed as mean ± SEM unless otherwise noted. Data were analyzed using One-way anova or t-tests using Graph Pad (Graph Pad Software Inc., CA, USA) for comparison of continuous variables. Differences were considered to be significant at p < 0.05.
Results
Emricasan treatment results in improved survival and protects from development of PHT induced by long-term BDL.
We performed bile duct ligation (BDL) on C57BL/6 mice, followed by an i.p injection with emricasan [IDN-6556] or placebo (Control) daily for 20 days at 10 mg/kg. Only animals that survived more than 5 days after BDL were included in the study because early deaths were attributed to the surgical procedure (Fig 1a). At 20 days, the gallbladder was obviously expanded in BDL groups compared to controls (Fig 1b). The livers showed macroscopic features of cholestasis in BDL groups compared to control groups (sham-placebo and sham-emricasan) while the gross consistency of the livers appeared different between the BDL-emricasan and BDL-placebo mice (Fig 1b). The liver/body ratio was significantly increased in those mice subjected to BDL (p<0.001), but no difference between BDL-placebo mice and BDL-emricasan mice was observed (Fig 1c). Notably, nearly all BDL-emricasan mice survived compared to BDL-placebo mice (p<0.05) over the 20 days period post BDL (Fig 1d). The survival rate was 75% in BDL-emricasan group, while it was 36% in BDL-placebo group (Fig 1d).
Fig. 1. Survival was improved in BDL treated with emricasan in long-term BDL.
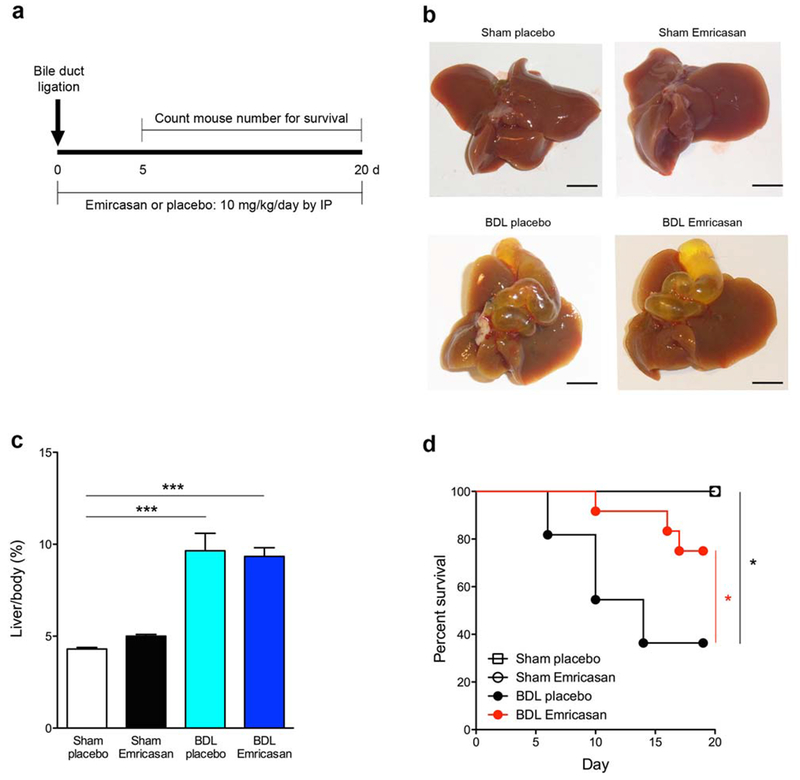
(a) Experimental design. (b) Liver pictures in control group and BDL group. Scale bar, 50 mm. (c) ratio of liver weight / body weight in control group and BDL group. ***p<0.001. (d) Survival curve in control group and BDL group *p<0.05. Values are mean ± SEM.
The development of PHT is one of the key triggers of complications resulting in increased mortality associated with cirrhosis. The improvement of BDL mouse survival with emricasan led us to investigate whether portal pressure is decreased in BDL-emricasan group. Before we measured the portal pressure, we observed macroscopically the expansion of the superior mesenteric vein (SMV), which combines with the splenic vein to form the hepatic portal vein. Indeed, SMV was expanded in BDL group compared to control group (Fig. 2a). SMV expansion was lower in BDL-emricasan mice compared to BDL-placebo mice (Fig. 2a). Portal pressure determination showed a significant increase in BDL-placebo mice compared to sham-placebo mice (p<0.001) (Fig. 2b). In contrast, BDL-emricasan mice had significantly lower levels of portal pressure than BDL-placebo mice (p<0.05) (Fig. 2b). Histopathologic examination of livers demonstrated bile ductular proliferation, portal edema, and mild portal infiltrates in BDL-placebo and BDL-emricasan mice (Fig. 2c). Serum ALT levels were significantly increased in BDL-placebo compared to control-placebo (p<0.01), while ALT levels were lower in BDL-emricasan mice compared to BDL-placebo mice although ALT levels were still higher in BDL emricasan compared to sham-placebo (p<0.05) (Fig. 2d). Although there was a trend towards a decrease in liver damage (fibrosis, cell death, and cholangiocyte proliferation), and in the circulating levels of MPs, these changes were not statistically significant between BDL-placebo and BDL-emricasan groups (supplementary Fig. 1,and 2)
Fig. 2. Portal pressure was decreased in BDL treated with emricasan in long-term BDL.
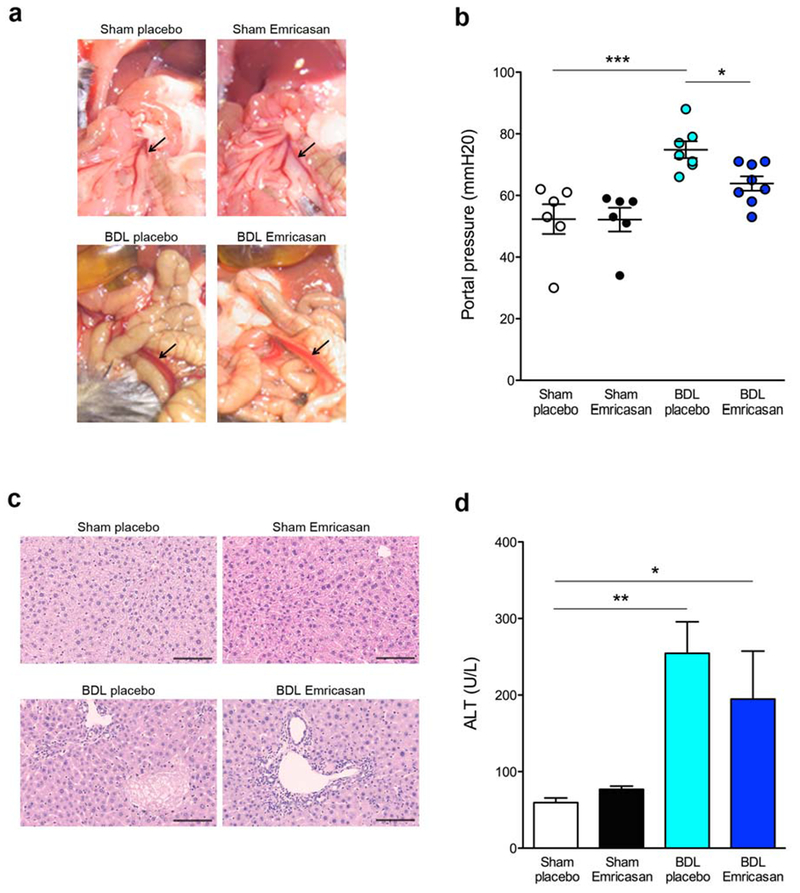
(a) Area of superior mesenteric vein (SMV) in control group and BDL group. Arrow shows SMV. (b) Portal pressure in control group and BDL group. *p<0.05, ***p<0.001. (c) Haematoxylin-eosin staining of liver sections in BDL-placebo mice and BDL-emricasan mice. Scale bar, 100 μm. (d) Liver ALT in control group and BDL group. *p<0.05, **p<0.01. Values are mean ± SEM.
Effects of emricasan treatment in short-term BDL.
In order to further explore the potential role of liver fibrosis and release of MPs as potential mechanism involved in the protective effects observed with emricasan therapy on PHT after prolonged BDL, we next examined an earlier time point by performing short-term BDL (10 days). The liver/body ratio was significantly increased in BDL groups compared to control groups (p<0.001), but no difference between BDL-placebo mice and BDL-emricasan mice (Fig. 3a). Histopathologic examination of livers demonstrated bile ductular proliferation, portal edema, and mild portal infiltrates in BDL-placebo and BDL-emricasan mice, indicating similar ductular responses of the liver to large bile duct obstruction in all groups (Fig. 3b). While confluent foci of hepatocyte death due to bile acid cytotoxicity, bile infarcts were reduced in BDL-emricasan mice (Fig. 3b). Liver fibrosis was significantly increased in BDL group compared to control group (p<0.001), however the extent of fibrosis was significantly lower in BDL-emricasan compared to BDL-placebo mice (Sirius red staining: p<0.05) (Fig. 3c, 3d and 3e). Liver angiogenesis was slightly decreased in BDL-emricasan compared to BDL-placebo mice (supplementary Fig. 3). Furthermore, cholangiocyte proliferation was significantly decreased in BDL-emricasan mice compared to BDL-placebo mice (p<0.05) (Fig. 3c and 3f). In addition, liver cell death assessed by TUNEL staining showed cell death was significantly improved in BDL-emricasan mice compared to BDL-placebo mice (p<0.05). (Fig. 3c and 3g). Next, we accessed the circulating MPs in blood via flow cytometry (Fig. 3h). The fold change of total MP number in the blood was significantly decreased in BDL-emricasan mice compared to BDL-placebo mice (p<0.001) (Fig. 3i).
Fig. 3. Circulating MPs were decreased in BDL treated with emricasan in short-term BDL.
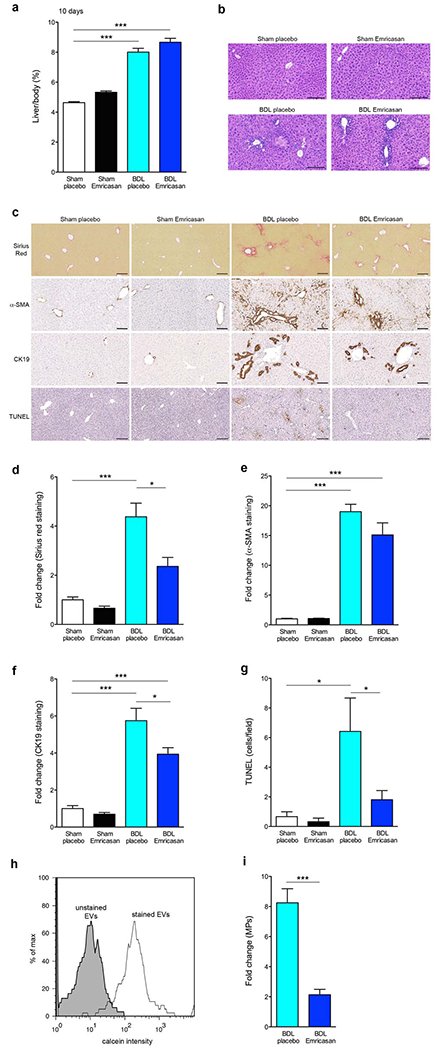
(a) Ratio of liver weight / body weight in control group and BDL group. ***p<0.001. (b) Haematoxylin-eosin staining of liver sections in control group and BDL group. Scale bar, 100 μm. (c) Sirius Red staining, α-SMA staining, CK19 staining, and TUNEL staining of liver sections in control group and BDL group. Scale bar, 100 μm. (d) Bar graph shows fold change of Sirius Red from the liver section stained with Sirius Red staining. *p<0.05, ***p<0.001. (e) Bar graph shows fold change of α-SMA from the liver section stained with α-SMA staining. **p<0.01, ***p<0.001. (f) Bar graph shows fold change of CK19 from the liver section stained with CK19 staining. *p<0.05, ***p<0.001. (g) Bar graph shows quantification of positive area of TUNEL from the liver section stained with TUNEL staining. *p<0.05. (h) Flow cytometry analysis of circulating MPs with dye from BDL mice. The black line represents labeled circulating MPs, whereas the gray area represents un-labeled circulating MPs (background control). (i) fold change of circulating MPs in BDL-placebo and BDL-emricasan compared to sham-placebo and sham-emricasan, respectively. *p<0.05, ***p<0.001. Values are mean ± SEM.
Differential functional effects of circulating MPs following BDL on endothelial cell activation.
Since we observed a significant reduction of circulating MPs following short-term BDL-in the emricasan-treated mice compared to BDL-placebo mice, we further investigated changes in MP effects on endothelial cell activity using HUVEC. We hypothesize that the increase in MPs following BDL might contribute to the development of PHT via modulation of endothelial cell activity. Labeled MPs with PKH26 were internalized by the entire HUVEC, which were stained by DAPI and F-actin, population within 4 h of incubation at 37°C as assessed by both flow cytometry (Fig. 4a) and fluorescent microscopy (Fig. 4b). HUVEC were incubated with purified circulating MPs from each group of mice and HUVEC activation was assessed via tube formation assays. HUVECs were activated by circulating MPs from BDL-placebo mice compared to circulating MPs from sham-placebo mice (p<0.001) and the activity was significantly reduced with circulating MPs from BDL-emricasan mice compared to BDL-placebo mice (p<0.001) (Fig. 4c and 4d). Circulating MPs from BDL-placebo mice contained VEGF, suggesting MP composition involved HUVEC activation (Fig. 4e).
Fig. 4. Circulating MPs from short-term BDL treated with emricasan reduced endothelial cell activity.
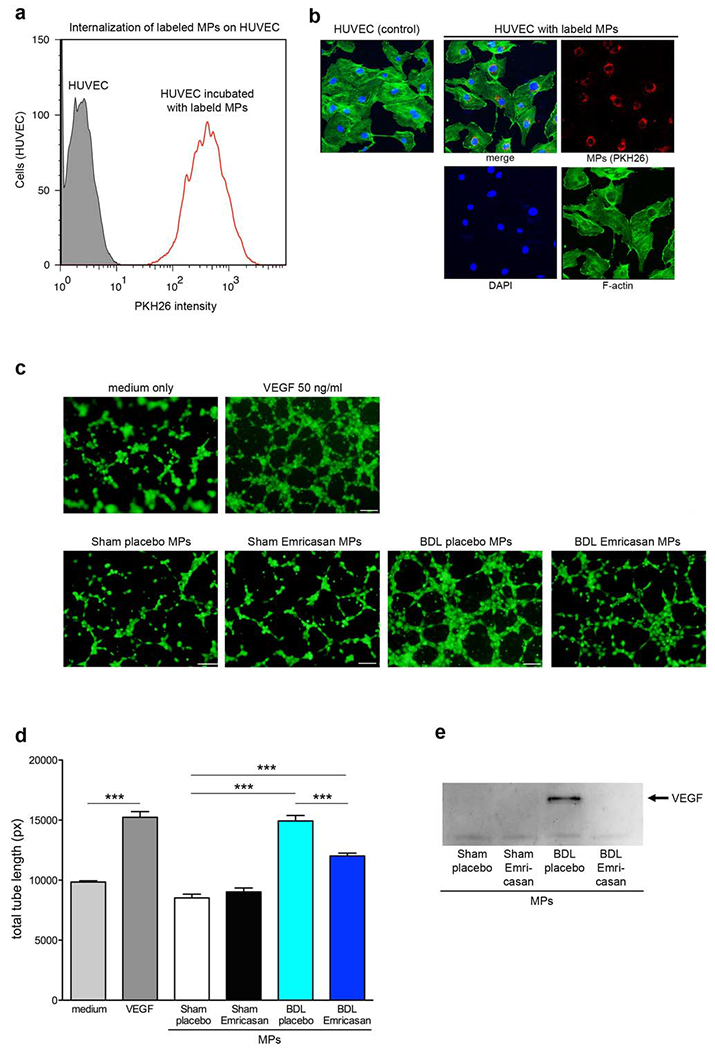
Internalization of labeled circulating MPs on HUVEC (a) by flow cytometry or (b) by confocal microscopy. (a) The red line represents HUVECs reacted with labeled circulating MPs, whereas the gray area represents HUVECs (background control). (b) Confocal image of HUVEC incubated with PKH26 (red) labeled circulating MPs. HUVEC was stained by DAPI (blue) for nuclei and by F-actin antibody (green). (c) Microscopy image of tube formation on HUVEC stained via calcein-AM. HUVEC were treated with medium (negative control), VEGF (positive control), or circulating MPs. (d) Bar graph shows quantification of total tube length. ***p<0.001. Values are mean ± SEM. (e) Western blot analysis of VEGF levels in MPs isolated from control or BDL mice.
Discussion
The main findings of the present study relate to the effects of emricasan, a pan-caspase inhibitor, on PHT and survival after BDL. The results demonstrated that treatment with emricasan reduced PHT and improved survival after long-term BDL and these effects were associated with decreased liver fibrosis and circulating vasoactive MPs. The result supports a potential role for pan-caspase inhibition in the treatment of PHT.
Caspase activation has been increasingly recognized as a central mediator of cell death during liver injury [11]. Targeting caspase activity has gained significant attention for development of novel therapeutic strategies for patients with various chronic liver diseases in particular those with NASH, a condition that is rapidly becoming a major cause of advanced liver disease [12–16]. Three independent pre-clinical studies using different models of dietary induced NASH in mice showed significant protective effects of pan-caspase inhibitors in parameter of fibrosis [8–10]. In addition to the effects of pan-caspase inhibition on modulation of fibrogenic pathways, it has been recently shown to modulate the generation and release of hemodynamically-active microparticles (MPs) [17]. Indeed, MPs are small membrane-bound particles released from dying or activated cells in a process involving caspase 3 and Rho-associated kinase activation [4, 5]. Studies in patients with cirrhosis have demonstrated that MPs are increased in circulation of these patients and impair vasoconstrictor responses contributing to arterial vasodilatation associated with portal hypertension [6]. In the present study, using murine model of portal hypertension, we tested the hypothesis that pan-caspase inhibition could led to a lowering of portal pressure via its anti-fibrotic effects and through inhibition of MP formation and release.
Emricasan treatment in mice after short-term BDL resulted in a significant improvement of liver damage, liver fibrosis, and liver cell death, both in the current study and a previous report [7]. Thus, suppression of liver fibrosis is one of the potential mechanisms for reduction of PHT. However, the suppression of liver fibrosis was not significant in mice after long-term BDL, suggesting that other mechanisms might be involved in the reduction of PHT. Recently, we have reported that hepatocyte-derived MPs were increased in circulation and were linked to angiogenesis in a murine NASH model [18] in part by modulating the activation of endothelial cells [19]. Furthermore, our group and other groups demonstrated that EV release was associated with caspase activation including caspase 3 and caspase 8 activations [4, 5, 17, 19, 20]. These results led us to further investigate whether emricasan, pan-caspase inhibitor, attenuated MP release resulting in suppression of endothelial cell activity. In long-term (20 days) BDL model, circulating MP number in BDL-emricasan trends toward a decrease compared to BDL-placebo, but overall, the number of circulating MP was only slightly up-regulated in BDL group compared to control group. Future studies to better determine the kinetics of MPs formation and release during chronic liver injury induced after BDL is warranted but it is tempting to speculate that following a prolonged period after BDL there is a decrease in disease activity, cell death, and decreased levels of MP release compared to the earlier stages of disease. As expected, in short-term BDL, circulating MPs were significantly up-regulated in BDL group about eight-fold compared to controls. Interestingly, MPs from BDL mice significantly activated endothelial cells. Notably, circulating MP number was significantly decreased in BDL-emricasan corresponding to significant suppression of liver cell death, resulting in a reduction of endothelial cell activity. These results suggest that the protective effects of emricasan treatment on PHT might be at least in part via modulation of MP-induced endothelial cell activation. Indeed, a recent pilot study in patients with advanced liver disease who were treated with emricasan showed reduced PHT by this treatment [21]. Future studies in additional experimental models as well as in patients with cirrhosis and PHT to further assess the effects of emricasan on MP release and their hemodynamic effects in the context of PHT are warranted.
In summary, the present study showed that in a murine model of long-term common bile-duct ligation, survival and PHT are improved by therapy with emricasan, a pan-caspase inhibitor, and in a murine model of short-term BDL, circulating MPs number and endothelial cell activity are decreased with emricasan treatment. emricasan is a promising agent for the treatment of PHT.
Supplementary Material
Emricasan, a pan-caspase inhibitor improves survival and portal hypertension induced by long-term Bile Duct Ligation (BDL) in mice
Emricasan reduces liver damage, hepatocyte death and fibrosis, following short-term BDL in mice and these changes are associated with a decrease in circulating microparticle (MPs)
Circulating MPs from BDL-placebo but not from BDL-emiricasan treated mice activate endothelial cells ex-vivo
Acknowledgements
UCSD Neuroscience Core for microscopy is supported by a grant NS047101.
Funding: This work was funded by NIH grants U01 AA022489 and Conatus Pharmaceuticals
Abbreviations:
- PHT
portal hypertension
- BDL
bile duct ligation
- MP
microparticle
Footnotes
Conflict of Interest: The authors state no conflict of interest, except Al Spada and Patricia Contreras are employees of Conatus Pharmaceuticals.
References
- 1.Ho SB, Matheny ME, Schnabl BE (2016) Changes in Hospital Admissions and Mortality for Complications of Cirrhosis: Implications for Clinicians and Health Systems. Gut and liver 10: 8–9. DOI 10.5009/gnl15593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laleman W, Trebicka J, Verbeke L (2016) Evolving insights in the pathophysiology of complications of cirrhosis: The farnesoid X receptor (FXR) to the rescue? Hepatology 64: 1792–1794. DOI 10.1002/hep.28771 [DOI] [PubMed] [Google Scholar]
- 3.Yanez-Mo M, Siljander PR, Andreu Z, Zavec AB, Borras FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J, et al. (2015) Biological properties of extracellular vesicles and their physiological functions. Journal of extracellular vesicles 4: 27066 DOI 10.3402/jev.v4.27066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eguchi A, Lazaro RG, Wang J, Kim J, Povero D, Willliams B, Ho SB, Starkel P, Schnabl B, Ohno-Machado L, et al. (2017) Extracellular vesicles released by hepatocytes from gastric infusion model of alcoholic liver disease contain a MicroRNA barcode that can be detected in blood. Hepatology 65: 475–490. DOI 10.1002/hep.28838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verma VK, Li H, Wang R, Hirsova P, Mushref M, Liu Y, Cao S, Contreras PC, Malhi H, Kamath PS, et al. (2016) Alcohol stimulates macrophage activation through caspase-dependent hepatocyte derived release of CD40L containing extracellular vesicles. Journal of hepatology 64: 651–660. DOI 10.1016/j.jhep.2015.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rautou PE, Bresson J, Sainte-Marie Y, Vion AC, Paradis V, Renard JM, Devue C, Heymes C, Letteron P, Elkrief L, et al. (2012) Abnormal plasma microparticles impair vasoconstrictor responses in patients with cirrhosis. Gastroenterology 143: 166–176 e166 DOI 10.1053/j.gastro.2012.03.040 [DOI] [PubMed] [Google Scholar]
- 7.Canbay A, Feldstein A, Baskin-Bey E, Bronk SF, Gores GJ (2004) The caspase inhibitor IDN-6556 attenuates hepatic injury and fibrosis in the bile duct ligated mouse. The Journal of pharmacology and experimental therapeutics 308: 1191–1196. DOI 10.1124/jpet.103.060129 [DOI] [PubMed] [Google Scholar]
- 8.Barreyro FJ, Holod S, Finocchietto PV, Camino AM, Aquino JB, Avagnina A, Carreras MC, Poderoso JJ, Gores GJ (2015) The pan-caspase inhibitor Emricasan (IDN-6556) decreases liver injury and fibrosis in a murine model of non-alcoholic steatohepatitis. Liver international : official journal of the International Association for the Study of the Liver 35: 953–966. DOI 10.1111/liv.12570 [DOI] [PubMed] [Google Scholar]
- 9.Witek RP, Stone WC, Karaca FG, Syn WK, Pereira TA, Agboola KM, Omenetti A, Jung Y, Teaberry V, Choi SS, et al. (2009) Pan-caspase inhibitor VX-166 reduces fibrosis in an animal model of nonalcoholic steatohepatitis. Hepatology 50: 1421–1430. DOI 10.1002/hep.23167 [DOI] [PubMed] [Google Scholar]
- 10.Anstee QM, Concas D, Kudo H, Levene A, Pollard J, Charlton P, Thomas HC, Thursz MR, Goldin RD (2010) Impact of pan-caspase inhibition in animal models of established steatosis and non-alcoholic steatohepatitis. Journal of hepatology 53: 542–550. DOI 10.1016/j.jhep.2010.03.016 [DOI] [PubMed] [Google Scholar]
- 11.Hirsova P, Gores GJ (2015) Death Receptor-Mediated Cell Death and Proinflammatory Signaling in Nonalcoholic Steatohepatitis. Cellular and molecular gastroenterology and hepatology 1: 17–27. DOI 10.1016/j.jcmgh.2014.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baskin-Bey ES, Washburn K, Feng S, Oltersdorf T, Shapiro D, Huyghe M, Burgart L, Garrity-Park M, van Vilsteren FG, Oliver LK, et al. (2007) Clinical Trial of the Pan-Caspase Inhibitor, IDN-6556, in Human Liver Preservation Injury. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 7: 218–225. DOI 10.1111/j.1600-6143.2006.01595.x [DOI] [PubMed] [Google Scholar]
- 13.Eguchi A, De Mollerat Du Jeu X, Johnson CD, Nektaria A, Feldstein AE (2016) Liver Bid suppression for treatment of fibrosis associated with non-alcoholic steatohepatitis. Journal of hepatology 64: 699–707. DOI 10.1016/j.jhep.2015.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lazic M, Eguchi A, Berk MP, Povero D, Papouchado B, Mulya A, Johnson CD, Feldstein AE (2014) Differential regulation of inflammation and apoptosis in Fas-resistant hepatocyte-specific Bid-deficient mice. Journal of hepatology 61: 107–115. DOI 10.1016/j.jhep.2014.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woolbright BL, Ding WX, Jaeschke H (2017) Caspase inhibitors for the treatment of liver disease: friend or foe? Expert review of gastroenterology & hepatology 11: 397–399. DOI 10.1080/17474124.2017.1300060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang P, Koyama Y, Liu X, Xu J, Ma HY, Liang S, Kim IH, Brenner DA, Kisseleva T (2016) Promising Therapy Candidates for Liver Fibrosis. Frontiers in physiology 7: 47 DOI 10.3389/fphys.2016.00047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eguchi A, Mulya A, Lazic M, Radhakrishnan D, Berk MP, Povero D, Gornicka A, Feldstein AE (2015) Microparticles release by adipocytes act as “find-me” signals to promote macrophage migration. PloS one 10: e0123110 DOI 10.1371/journal.pone.0123110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Povero D, Eguchi A, Li H, Johnson CD, Papouchado BG, Wree A, Messer K, Feldstein AE (2014) Circulating Extracellular Vesicles with Specific Proteome and Liver MicroRNAs Are Potential Biomarkers for Liver Injury in Experimental Fatty Liver Disease. PloS one 9: e113651 DOI 10.1371/journal.pone.0113651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Povero D, Eguchi A, Niesman IR, Andronikou N, de Mollerat du Jeu X, Mulya A, Berk M, Lazic M, Thapaliya S, Parola M, et al. (2013) Lipid-induced toxicity stimulates hepatocytes to release angiogenic microparticles that require Vanin-1 for uptake by endothelial cells. Science signaling 6: ra88 DOI 10.1126/scisignal.2004512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirsova P, Ibrahim SH, Krishnan A, Verma VK, Bronk SF, Werneburg NW, Charlton MR, Shah VH, Malhi H, Gores GJ (2016) Lipid-Induced Signaling Causes Release of Inflammatory Extracellular Vesicles From Hepatocytes. Gastroenterology 150: 956–967. DOI 10.1053/j.gastro.2015.12.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia-Tsao G, Fuchs M, Shiffman ML, Chan JL, Morris M, Yamashita M, Spada AP, Hagerty D, Bosch J Emricasan (IDN-6556) administered orally for 28 days lowers portal pressure in patients with compensated cirrhosis and severe portal hypertension. Hepatology 62: 1382A. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


