Fig. 1.
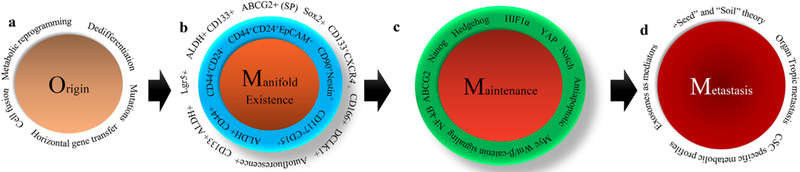
Overall journey of CSCs from origin to metastasis. a) Origin of CSCs. Mutations in adult stem cells (ASCs) or in differentiated somatic cells can lead to CSC origin. Dedifferentiation of somatic differentiated cell in response to various external toxic exposures can give rise to CSC phenotype. Other factors, such as metabolic reprogramming, cell fusion, and horizontal gene transfer can also induce CSCs. b) Multiple CSC populations reside within tumors. CSCs with detoxification systems such as ABCG2-mediated drug efflux mechanism and ALDH-mediated aldehyde toxic substance detoxification systems exist in various tumors. CSCs expressing cell surface markers such as CD44, CD24, and EpCAM together are also the major constituents within various heterogeneous tumors, such as pancreatic tumors. Other CSCs, which express CD133 and CXCR4, also reside within the same tumor. Intestinal tumors consist of Lgr5-expressing CSCs. c) ‘Stemness’ maintenance mechanisms. The stemness in CSCs is largely maintained by specific stemness molecules such as Wnt/β-catenin, Notch and hedgehog, along with other factors such as YAP, HIF1α, NF-kB, PPARγ, and antiapoptotic. d) Role of CSCs in metastasis. The “seed” and “soil” theory, as proposed by Stephen Paget, states that primary site tumor cells (seed) travel to a distant organ (soil), and colonize and initiate the growth of tumor. Based on this theory, it is possible that CSCs from the primary site will travel to distant organs to initiate metastatic tumors. Another hypothetical view suggests that exosomes released by CSCs in the primary site travel to target sites and form the premetastatic niche (PMN) that supports upcoming CSCs or cancer cells. Another view also suggests that distinct CSC population subtypes with subtype-specific metabolic profiles travel to different organs (organ specific metastasis).
