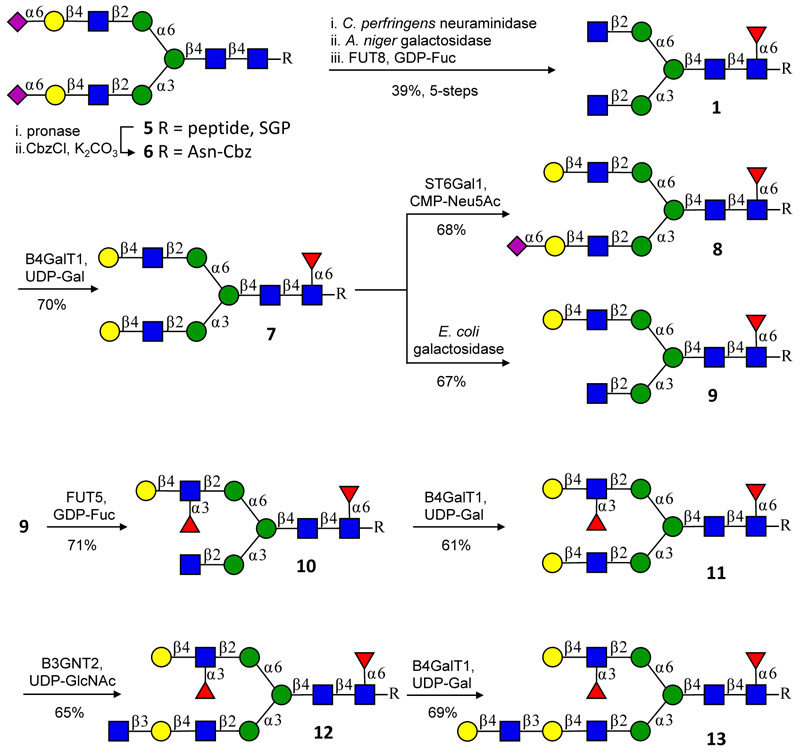
Fig. 2.
Two strategies for desymmetrizing N-glycans using the branch selectivity of the sialyltransferase ST6Gal1 and the galactosidase from E. coli, and subsequent preparation of asymmetric branched bi-antennary glycans such as 13. The α2,6-sialoside of 8 blocks further modification of the MGAT1 antenna allowing selective elaboration of the MGAT2 arm. The MGAT1 and MGAT2 arms of asymmetrically branched glycan 9 can selectively be extended by exploiting that many glycosyltransferases modify LacNAc but not terminal GlcNAc moieties making it was possible to first elaborate the MGAT2 arm without affecting the MGAT1 arm. The peptide sequence of SGP is NH2-Lys-Val-Ala-Asn-Lys-Thr-COOH with the glycan connected to Asn. Each intermediate was purified by HPLC on an XBridge HILIC column. The transformation of 10 into 13 was also performed without intermediate compound purification and by only subjecting 13 to P2 size exclusion column chromatograph, an improved yield of 79% over three steps was accomplished.