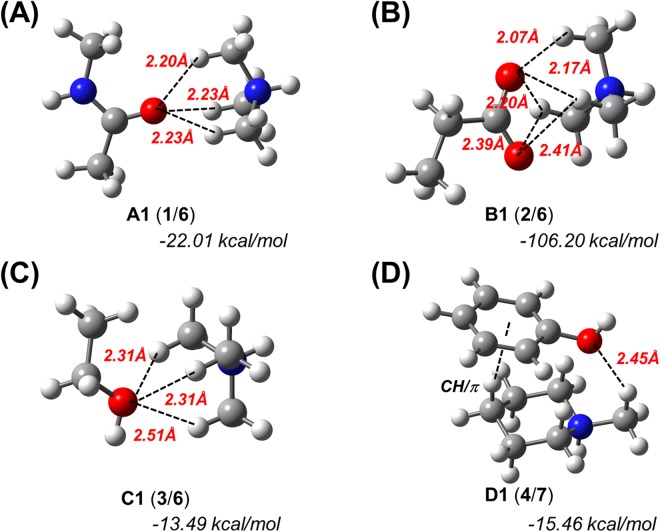
Figure 1.
Theoretically optimized geometries and counterpoise-corrected interaction energies for N+-C-H··O hydrogen bond models. The geometry optimizations and energy calculations were carried out at the M06-2X/6-311++G** level of theory in the gas phase. (A) Trimethylammonium (6) complexed with N-methylacetamide (1). (B) 6 complexed with propanoate (2). (C) 6 complexed with ethanol (3). (D) N-methylpiperidium (7) complexed with phenol (4).