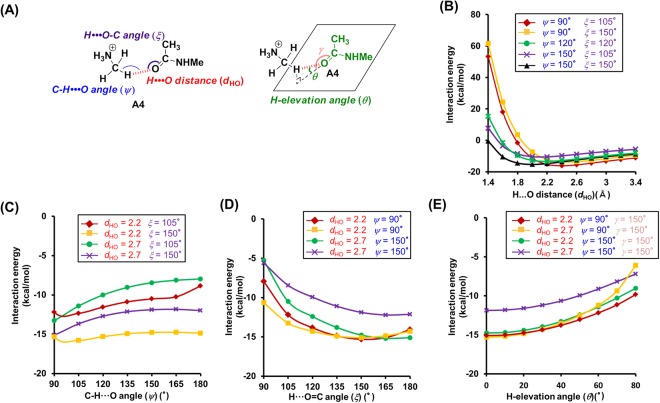
Figure 2.
Theoretical analysis of the dependence of the interaction energies on the geometry of the N+-C-H···O hydrogen bonds between N-methylacetamide (1) and monomethylamine (5). (A) Geometry of the N+-C-H···O hydrogen bond model between 1 and 5. (B–E) Interaction energies of the N+-C-H···O hydrogen bond as a function of the following geometry parameters. (B) H···O distance (dHO). (C) C-H···O angle (ψ). (D) H···O=C angle (ξ). (E) H-elevation angle (θ). For the analysis of the H···O distance, the C-H···O, H···O=C, and H-elevation angles were kept constant (0°). Interaction energies were corrected for basis set superposition error (BSSE) by counterpoise correction.