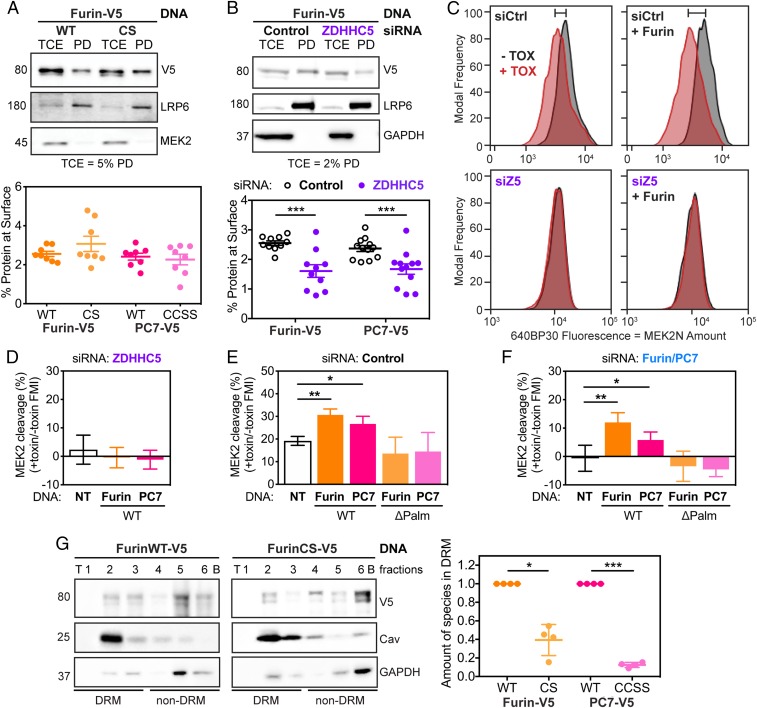
Fig. 4.
Palmitoylation-deficient mutants of Furin and PC7 are not active in cleaving anthrax toxin because they do not preferentially reside in microdomains. (A) RPE-1 cells were transfected with WT or palmitoylation-deficient mutants of Furin (shown) or PC7 (in SI Appendix, Fig. S5A). The cells were biotinylated for 30 min and then quenched, washed, and lysed. The surface fraction was pulled down using streptavidin beads. SDS/PAGE was performed on TCE and pull-down (PD) fractions with the percent of TCE shown. Statistics: unpaired two-tailed t test. (B) As in A, RPE-1 cells were transfected with V5-tagged Furin (shown) or PC7 (shown in SI Appendix, Fig. S5B) after being silenced for ZDHHC5 or not (Control). Then, the surface proteins were labeled with biotin and isolated. Statistics: unpaired two-tailed t test. (C) Analytical flow cytometry was performed as described in Fig. 1 but with Furin overexpression (using an antibody against V5 to select for transfected cells) or not in cells silenced for Control (Ctrl) or ZDHHC5 (Z5). The shift in MEK cleavage between no toxin (−TOX, black) and toxin (+TOX, red) is noted by the bar on top of the graph. Quantification of recomplementation flow cytometry peaks as in C with overexpression of both Furin and PC7, WT or palmitoylation-deficient mutants (ΔPalm), in ZDHHC5- (D), Control- (E), and F/PC7- (F) silenced cells. Statistics: paired two-tailed t test on the original data. (G) OptiPrep ultracentrifugation gradients were used to probe presence of Furin, WT or CS mutant in DRMs. Representative blot with DRM control (caveolin 1) and non-DRM control (GAPDH) for Furin (shown) or PC7 (shown in SI Appendix, Fig. S5G). Statistics: ratio paired two-tailed t test on the original data. *P < 0.05, **P < 0.01, and ***P < 0.001.