Significance
The debate on the onset of plate tectonics in the Earth’s history has partially originated from the controversial criteria of using felsic crust to trace plate tectonics in the past. Here, we demonstrate how Ti isotope ratios can be used as a proxy for the affinity of felsic rocks to plume or island arc settings. Our study shows that, contrary to what was previously assumed, Ti isotopes cannot serve as a direct evidence for plate tectonics from 3.5 billion years ago, and must be combined with other information on SiO2 contents of crustal rocks to be reliable.
Keywords: titanium isotopes, plume, island arc, magma differentiation, plate tectonics
Abstract
Indirect evidence for the presence of a felsic continental crust, such as the elevated 49Ti/47Ti ratios in Archean shales, has been used to argue for ongoing subduction at that time and therefore plate tectonics. However, rocks of intermediate to felsic compositions can be produced in both plume and island arc settings. The fact that Ti behaves differently during magma differentiation in these two geological settings might result in contrasting isotopic signatures. Here, we demonstrate that, at a given SiO2 content, evolved plume rocks (tholeiitic) are more isotopically fractionated in Ti than differentiated island arc rocks (mainly calc-alkaline). We also show that the erosion of crustal rocks from whether plumes (mafic in average) or island arcs (intermediate in average) can all produce sediments having quite constant 49Ti/47Ti ratios being 0.1–0.3 per mille heavier than that of the mantle. This suggests that Ti isotopes are not a direct tracer for the SiO2 contents of crustal rocks. Ti isotopes in crustal sediments are still a potential proxy to identify the geodynamical settings for the formation of the crust but only if combined with additional SiO2 information.
The onset of plate tectonics is still highly debated due to the fragmentary geologic record for the early Earth. The proposed onset time of plate tectonics in literature has ranged from >4.2 to 0.85 billion years ago (Ga), and the emergence of the felsic continental crust has been usually considered as a major proxy to trace plate tectonics in the past (1–8). Nonetheless, the validity of such a criteria to trace the onset of plate tectonics has been frequently questioned based on the fact that both plume (tholeiitic) and island arc (mainly calc-alkaline) settings are able to produce rocks of intermediate to felsic compositions (9–11). Thus, it is critical to find a geochemical proxy to differentiate the plume or island arc affinity of felsic rocks in the past.
Titanium isotopes have been recently proposed to be a direct tracer for the SiO2 contents of crustal rocks, based on the monotonic correlation between the δ49Ti values (the per mille deviation of the 49Ti/47Ti ratio relative to Origins Laboratory Ti standard) and SiO2 contents of differentiated island arc rocks (1). However, this proposal is yet to be confirmed since Ti isotopes can be expected to follow contrasting isotope systematics during magma differentiation in plume and island arc settings. For instance, plume lavas (H2O-poor and reduced) have a higher solubility of Fe-Ti oxides than island arc lavas (H2O-rich and oxidized), thus developing enrichments in both total Fe and TiO2 contents during fractional crystallization of olivine and plagioclase (12–14), potentially leading to variable Ti isotopic fractionation of the melts during fractional crystallization of Fe-Ti oxides.
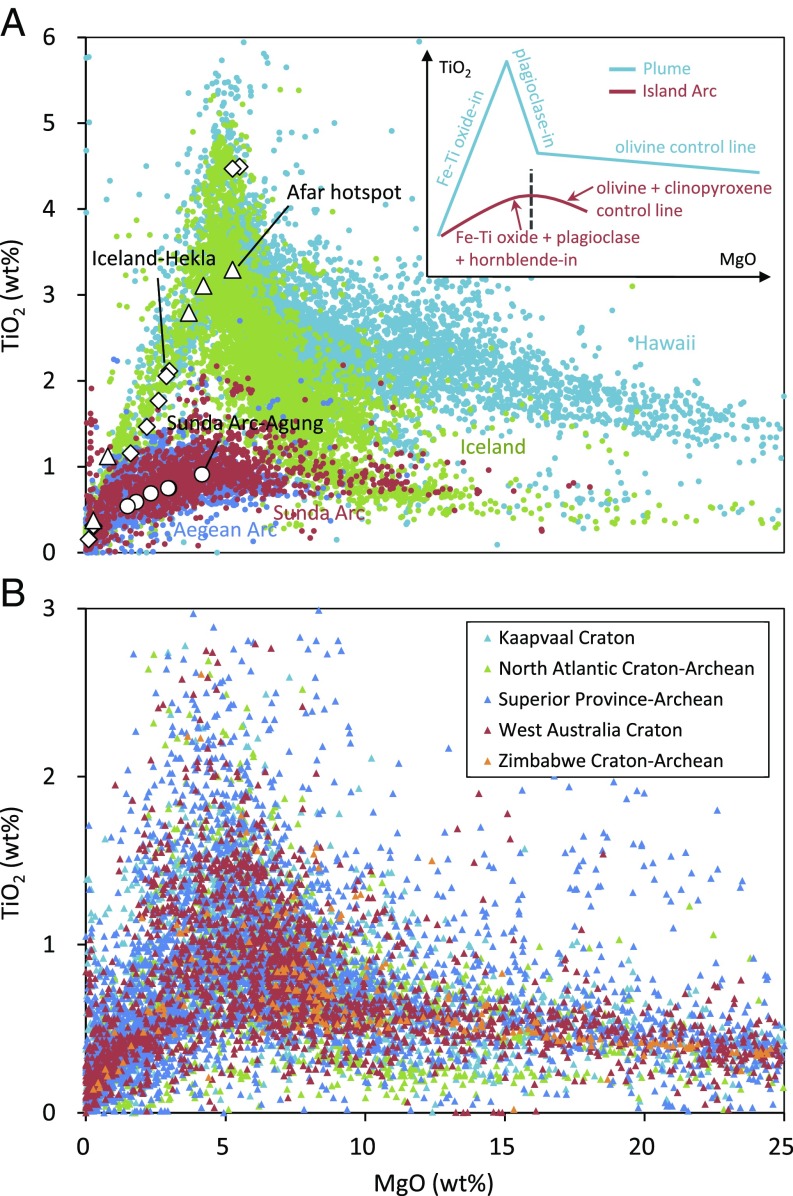
Here, we report the Ti isotopic composition of a set of well-characterized rocks from two typical plume settings, the Hekla volcano in Iceland (15) and the Afar hotspot in East Africa (16). These data are compared with data from a typical arc setting (Agung volcano, Sunda Arc) (17), to establish the systematics for the isotopic behavior of Ti during magmatic differentiation. The Hekla/Afar and Agung rocks were chosen because they are good analogs to document the magmatic behaviors of Ti isotopes during the generation of the Archean crust. First, the ranges in major element composition for Hekla/Afar and Agung samples encompass the compositional ranges known for present-day plume and island arc settings, respectively (Fig. 1A). Second, rocks from Archean cratons have a TiO2 versus MgO pattern similar to that shown by present-day rocks from plume and island arc settings: tholeiitic rocks show an enrichment in TiO2 during magma differentiation, whereas calc-alkaline rocks do not (Fig. 1B). The systematics established for Ti isotopes in Hekla/Afar and Agung rocks allow to model δ49Ti values of Archean crustal rocks from plume and island arc settings. These distributions are compared with Ti isotopic data on a large set of Archean sediments [i.e., banded iron formation (BIF), cherts, and shales with ages from ∼3.8 to ∼0.45 Ga] that were also studied to identify the geodynamical origin of the Archean continental crust.
Fig. 1.
The TiO2 and MgO contents in the rocks from present-day plume (Iceland and Hawaii) and island arc (Sunda and Aegean Arcs) settings (A) and from Archean cratons (B). The chemical composition data of these rocks are from the GeoRoc database. The samples from Hekla (Iceland) (15), Afar hotspot (East Africa), and Agung (Sunda Arc) (19) are shown in A. The Inset in A illustrates the typical fractionation path inducing the TiO2 variations in plume lavas (18) and island arc lavas (14).
Results
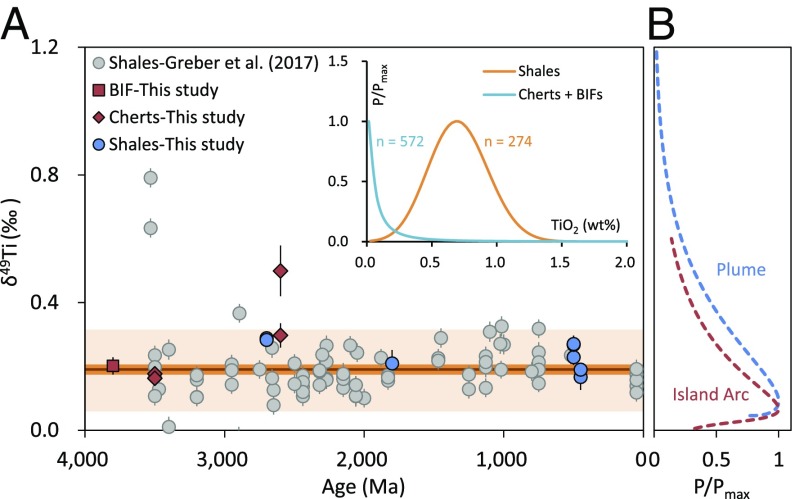
The Hekla and Afar samples cover all of the range in TiO2 contents typical of Fe-Ti oxide fractionation in a plume setting (15, 16, 18) (Fig. 1A). These samples are aphyric (<5% phenocryst) and show a wide lithological range from basalt to rhyolite with (i) progressive enrichments in SiO2, K2O, Rb, and Th and (ii) progressive depletions in MgO, total Fe, CaO, and TiO2 contents (15, 16). Their δ49Ti values vary from −0.005 ± 0.028‰ to +2.012 ± 0.014‰. In addition, δ49Ti values are strongly correlated with chemical parameters such as TiO2, total Fe, and SiO2 contents (Fig. 2). On the other hand, the δ49Ti values of the Archean BIFs, cherts, and shales are less variable, with values from +0.163 ± 0.028‰ to +0.500 ± 0.080‰ (Fig. 3A). These BIF, chert, and shale samples are from rock formations that have been intensively studied (SI Appendix). Together with the Ti isotopic data for shales from literature (1), the present data confirm the systematically positive δ49Ti values for sediments since early Archean (Fig. 3A). The majority of samples have δ49Ti values of +0.079‰ to +0.366‰ (n = 88), with five samples showing values down to −0.018‰ or up to +0.791‰ (n = 5).
Fig. 2.
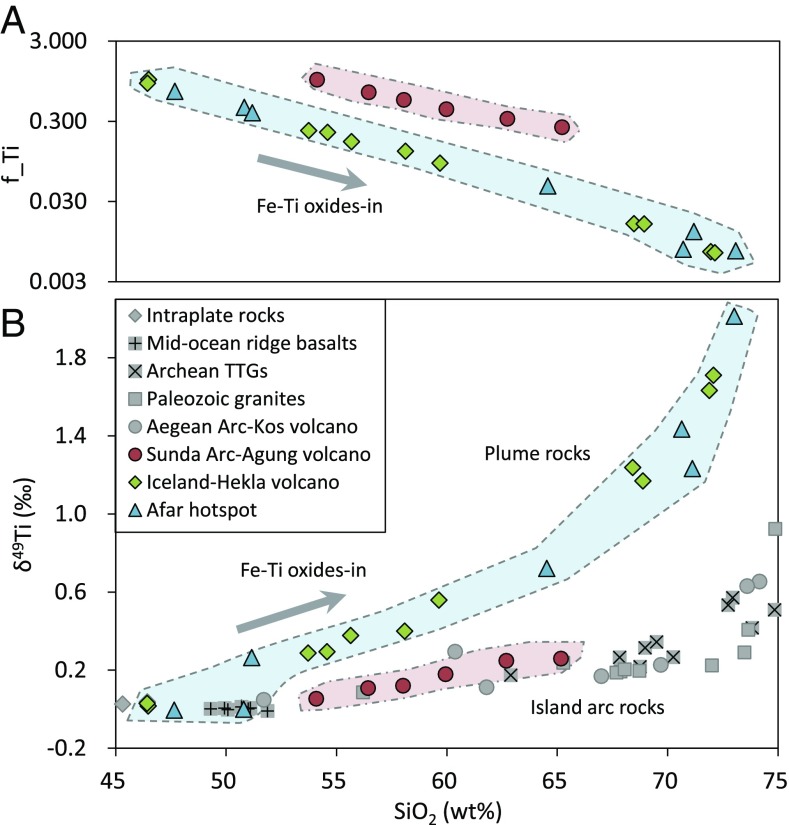
The fractions of Ti (f_Ti) remaining in the melts versus SiO2 contents (A) and the variations of δ49Ti values versus SiO2 contents (B) for the Hekla (15), Afar hotspot (East Africa), and Agung (17, 19) samples. The f_Ti values of the samples were estimated from their Ti/Rb ratios. The arrows indicate the effects from the fractional crystallization of Fe-Ti oxides, e.g., ilmenite, titanomagnetite, or titanite (14, 18). Literature data of Paleozoic granites, Archean TTGs, and volcanic rocks from the Kos volcano of Aegean Arc in ref. 1, as well as those of MORBs and intraplate rocks in ref. 17, are also shown for comparison. The errors on the δ49Ti values are 95% confidence intervals that are smaller than the size of the labels.
Fig. 3.
(A) The δ49Ti values of Archean BIF, cherts, and shales versus depositional ages and previous data for shales (1) (Dataset S7). The dark orange line shows the average δ49Ti value of ∼+0.191‰ for the present data, with the orange and light orange areas showing 2 SE (±0.013‰) and 2 SD (±0.119‰) of these data, respectively. The Inset shows the distribution of the literature TiO2 contents in BIFs, cherts, and shales with the probabilities normalized to the maximal probability (P/Pmax) (Dataset S8). (B) Distribution of the δ49Ti values for plume and island arc rocks in the GeoRoc database. The samples were compiled into groups in each 0.5 wt % SiO2 interval following Gaussian distribution (SI Appendix, Fig. S2). This SiO2 distribution was further translated into the δ49Ti density based on the δ49Ti-SiO2 systematics for plume settings (this study) and island arc settings (1).
Discussion
The variations of δ49Ti values in the Hekla/Afar samples are correlated with TiO2, SiO2, and total Fe in a manner indicating that they primarily reflect the fractional crystallization of Fe-Ti oxides during magma differentiation (Fig. 2). The compositions of Agung samples from Sunda Arc (17, 19) can be interpreted in the same way (Fig. 2). However, while the end points of differentiation for Hekla/Afar and for Agung seem to be grossly similar in TiO2 contents (Fig. 1A), the exact fractions of Ti remaining in the melt (f_Ti) at a given SiO2 content are in fact very different for the two magmatic suites (at 60 wt % SiO2 f_Ti, ∼0.087 and ∼0.213 for Hekla/Afar and Agung, respectively; Fig. 2A). These fractions (f_Ti) can be estimated from the Ti/Rb ratio: Rb being strongly incompatible allows to correct for the change in TiO2 content due to fractionation of Ti-free major silicate phases (Fig. 1A, Inset). The fractional crystallization of ilmenite or titanomagnetite results in an increase of the δ49Ti value of the melts (up to ∼+2.012‰ for Hekla/Afar) during magma differentiation. Modeling of the correlation between δ49Ti and f_Ti shows that the Ti isotopic fractionation between crystals (ilmenite or titanomagnetite) and melt (noted Δ49Ticrystal-melt) increases during magma differentiation for the Hekla/Afar lavas (SI Appendix, Fig. S1), from ∼−0.1‰ at ∼1,500 K to ∼−0.5‰ at ∼1,150 K. This is likely not just due to a change in temperature but also to a change in melt structure with increasing SiO2 contents, which results in an enhancement of the proportion of lower-coordinated Ti in silicate melts and of the Ti isotopic fractionation between oxides and melts. Hekla and Afar lavas follow very similar δ49Ti-SiO2 paths, even though these two igneous suites have quite different peak TiO2 contents (4.53 wt % for Hekla and 3.30 wt % for Afar). This confirms that the Ti stable isotopic behaviors during magma differentiation are primarily controlled by the f_Ti values but not the TiO2 contents. While the Agung samples document a more limited compositional range (17), the calculated Δ49Ticrystal-melt value for these samples is similar to the values determined for the Hekla/Afar samples. The fundamental reason for the different δ49Ti versus SiO2 trends between plume and arc settings is that the plume lavas experience much larger fractionations of Ti after the saturation of Fe-Ti oxides due to (i) the lower fO2 values delaying ilmenite or titanomagnetite saturation and allowing the enrichments of TiO2 in magmas by olivine and plagioclase accumulation (12, 18) (Fig. 1A, Inset) and (ii) the higher TiO2 contents in the magmas allowing larger magnitudes of fractionation of Ti by fractional crystallization of Fe-Ti oxides (13) (Fig. 2A). Therefore, the contrasting Ti stable isotopic behaviors between plume and island arc rocks suggest that Ti isotopes cannot be used as a direct tracer for the SiO2 contents of the crustal protoliths of Archean sedimentary rocks, contrary to that recently proposed in ref. 1. This is because δ49Ti values in the range from ∼0.0‰ to ∼+0.4‰ can be reached at very different SiO2 contents on the plume and island arc trends (Fig. 2B). Ti isotopes are useful to discriminate whether crustal rocks are from plume or island arc settings only when they reach δ49Ti values ≥0.4‰, i.e., the felsic ends of the two trends (Fig. 2B).
It is worth noting that, despite being built on samples from present-day plume and island arc settings, the present δ49Ti-SiO2 proxy for the tholeiitic or calc-alkaline affinity of felsic rocks can be also applicable for Archean rocks. In the TiO2 versus MgO plot (Fig. 1B), the rocks from Archean cratons exhibit magma differentiation paths very similar to those of present-day lavas, i.e., the enrichments of TiO2 in plume-type (tholeiitic) rocks during the fractional crystallization of olivine and plagioclase and the lack of such a TiO2 enrichment in island arc-type (calc-alkaline) rocks (Fig. 1A). The Archean rocks only differ from the present-day lavas in a reduction of the maximum range in TiO2 content, as a consequence of the higher degrees of mantle partial melting in the Archean (20). This is unlikely to significantly affect the isotopic behavior of Ti since the evolution of δ49Ti values during magma differentiation is primarily controlled by the fraction of Ti remaining in the melt (f_Ti), as corroborated by the similar δ49Ti-SiO2 paths for Hekla and Afar igneous suites having quite different peak TiO2 contents (Fig. 2).
While shales show high TiO2 contents with an average value of ∼0.63 wt %, the BIFs and cherts are quite depleted in Ti with TiO2 ≤ 0.01 wt % for the majority of samples (Fig. 3A, Inset and Dataset S8). Such depletions in Ti for BIFs and cherts are the results of the enrichments of other elements during chemical precipitation processes (e.g., Si for cherts), thus modifying the proportions of detrital Fe-Ti oxides. Nonetheless, the δ49Ti values of the present BIFs and cherts are similar to those of shales in this study and the literature (1) (Dataset S7). The similar δ49Ti between low- and high-TiO2 sedimentary rocks confirms the robustness of Ti isotopes in tracing the composition of their protoliths. It is striking that the δ49Ti values of BIFs, cherts, and shales since 3.8 Ga have been rather uniformly 0.1–0.3‰ higher than the typical mantle value of ∼0‰ [e.g., mid-ocean ridge basalts (MORBs)] (17, 21), with an average of +0.191 ± 0.013‰ (2 SE; n = 88) (1). This can, however, be simply explained from considerations on the likely distributions of δ49Ti values in plume and island arc rocks. These distributions can be modeled in Fig. 3B from the distribution of SiO2 contents (extracted from the GeoRoc database; SI Appendix, Fig. S2) and from the relation established between SiO2 and δ49Ti (Fig. 2B). The result shows that both plume (in average SiO2, ∼50 wt %) and island arc settings (in average SiO2, ∼59 wt %), and Archean tonalite–trondhjemite–granodiorite rocks (TTGs) or Paleozoic granites with SiO2 of ∼65–70 wt % (Dataset S6), can develop δ49Ti values close to the values of Archean sedimentary rocks. Thus, the uniform δ49Ti value of Archean sediments at ∼+0.2‰ is not a solid argument for the presence of an early Archean felsic continental crust. Future studies of Archean sediments should aim at combining Ti isotopes with reliable information of SiO2 contents of their protoliths, to differentiate safely a plume from an island arc affinity of the continental crust in the past. For instance, since the modern continental crust is known to be in average “andesitic” in bulk composition [i.e., SiO2 = 60.6 wt %, with SiO2 = 63.5–66.6 wt % for its middle to upper parts (22)], the δ49Ti values of +0.1–0.3‰ for the Phanerozoic sedimentary rocks likely suggest an island arc origin of this continental crust.
Materials and Methods
The Hekla/Afar and sedimentary samples were crushed into powders with an agate mortar, and the rock powders were dissolved by HF-HNO3 digestion on a hotplate or by alkali fusion (15, 23). Sample aliquots were mixed with a 47Ti-49Ti double spike and were processed with a three-step ion-exchange chromatographic procedure, consisting of Eichrom N,N,N′,N′-tetra-n-octyldiglycolamide (DGA) resin and analytical grade anion exchange resin in chloride form and 8% cross-linkage (AG1-X8) (24), to purify Ti from the matrices. Ti isotopes were measured on a Thermo-Fisher Neptune multicollector inductively coupled plasma mass spectrometer (MC-ICP-MS) at the Institut de Physique du Globe de Paris with medium mass resolution (M/ΔM, ∼5,800). Samples were introduced in 0.5 M HNO3 plus 0.0015 M HF through an Apex desolvating nebulizer with a concentration of 300 ppb Ti. Signals of 46Ti, 47Ti, 48Ti, and 49Ti were used for double-spike inversion using the IsoSpike software (25). Full methods and associated references are available in SI Appendix.
Supplementary Material
Acknowledgments
We thank Pascale Louvat, Jessica Dallas and Pierre Burckel for the maintenance of the (MC)-ICP-MS at Institut de Physique du Globe de Paris (IPGP) and John Creech for providing updated IsoSpike. Paolo Sossi and Kirsten van Zuilen are appreciated for discussions. The editor and two anonymous reviewers are thanked for the comments, which significantly improved this manuscript. F.M. acknowledges funding from the European Research Council (ERC) under Horizon 2020 Framework Programme/ERC Grant Agreement 637503 (Pristine). F.M. and M.C. acknowledge the financial support of the UnivEarthS Labex Program at Sorbonne Paris Cité (Grants ANR-10-LABX-0023 and ANR-11-IDEX-0005-02). Parts of this work were supported by IPGP Plateau d’Analyse haute Résolution (PARI) and by Region Île-de-France Sesame Grant 12015908.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1809164116/-/DCSupplemental.
References
- 1.Greber ND, et al. Titanium isotopic evidence for felsic crust and plate tectonics 3.5 billion years ago. Science. 2017;357:1271–1274. doi: 10.1126/science.aan8086. [DOI] [PubMed] [Google Scholar]
- 2.Korenaga J. Initiation and evolution of plate tectonics on Earth: Theories and observations. Annu Rev Earth Planet Sci. 2013;41:117–151. [Google Scholar]
- 3.Watson EB, Harrison TM. Zircon thermometer reveals minimum melting conditions on earliest Earth. Science. 2005;308:841–844. doi: 10.1126/science.1110873. [DOI] [PubMed] [Google Scholar]
- 4.Martin H. Effect of steeper Archean geothermal gradient on geochemistry of subduction-zone magmas. Geology. 1986;14:753–756. [Google Scholar]
- 5.Dhuime B, Wuestefeld A, Hawkesworth CJ. Emergence of modern continental crust about 3 billion years ago. Nat Geosci. 2015;8:552–555. [Google Scholar]
- 6.Tang M, Chen K, Rudnick RL. Archean upper crust transition from mafic to felsic marks the onset of plate tectonics. Science. 2016;351:372–375. doi: 10.1126/science.aad5513. [DOI] [PubMed] [Google Scholar]
- 7.Harrison TM, Bell EA, Boehnke P. Hadean zircon petrochronology. Rev Mineral Geochem. 2017;83:329–363. [Google Scholar]
- 8.Boehnke P, et al. Potassic, high-silica Hadean crust. Proc Natl Acad Sci USA. 2018;115:6353–6356. doi: 10.1073/pnas.1720880115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell IH, Davies DR. Raising the continental crust. Earth Planet Sci Lett. 2017;460:112–122. [Google Scholar]
- 10.Reimink JR, Chacko T, Stern RA, Heaman LM. Earth’s earliest evolved crust generated in an Iceland-like setting. Nat Geosci. 2014;7:529–533. [Google Scholar]
- 11.Willbold M, Hegner E, Stracke A, Rocholl A. Continental geochemical signatures in dacites from Iceland and implications for models of early Archaean crust formation. Earth Planet Sci Lett. 2009;279:44–52. [Google Scholar]
- 12.Toplis MJ, Carroll MR. An experimental study of the influence of oxygen fugacity on Fe-Ti oxide stability, phase relations, and mineral-melt equilibria in ferro-basaltic systems. J Petrol. 1995;36:1137–1170. [Google Scholar]
- 13.Cawthorn RG, Biggar GM. Crystallization of titaniferous chromite, magnesian ilmenite and armalcolite in tholeiitic suites in the Karoo Igneous Province. Contrib Mineral Petrol. 1993;114:221–235. [Google Scholar]
- 14.Nandedkar RH, Ulmer P, Müntener O. Fractional crystallization of primitive, hydrous arc magmas: An experimental study at 0.7 GPa. Contrib Mineral Petrol. 2014;167:1015. [Google Scholar]
- 15.Savage PS, Georg RB, Williams HM, Burton KW, Halliday AN. Silicon isotope fractionation during magmatic differentiation. Geochim Cosmochim Acta. 2011;75:6124–6139. [Google Scholar]
- 16.Pik R, Marty B, Hilton DR. How many mantle plumes in Africa? The geochemical point of view. Chem Geol. 2005;226:100–114. [Google Scholar]
- 17.Millet MA, et al. Titanium stable isotope investigation of magmatic processes on the Earth and Moon. Earth Planet Sci Lett. 2016;449:197–205. [Google Scholar]
- 18.Prytulak J, Elliott T. TiO2 enrichment in ocean island basalts. Earth Planet Sci Lett. 2007;263:388–403. [Google Scholar]
- 19.Dempsey S. 2013. Geochemistry of volcanic rocks from the Sunda Arc. Doctoral thesis (Durham University, Durham, United Kingdom)
- 20.Keller CB, Schoene B. Statistical geochemistry reveals disruption in secular lithospheric evolution about 2.5 Gyr ago. Nature. 2012;485:490–493. doi: 10.1038/nature11024. [DOI] [PubMed] [Google Scholar]
- 21.Deng Z, Moynier F, Sossi PA, Chaussidon M. Bridging the depleted MORB mantle and the continental crust using titanium isotopes. Geochem Perspect Lett. 2018;9:11–15. [Google Scholar]
- 22.Rudnick RL, Gao S. Composition of the continental crust. Treatise Geochem. 2003;3:1–64. [Google Scholar]
- 23.Deng Z, et al. Lack of resolvable titanium stable isotopic variations in bulk chondrites. Geochim Cosmochim Acta. 2018;239:409–419. [Google Scholar]
- 24.Zhang J, Dauphas N, Davis AM, Pourmand A. A new method for MC-ICPMS measurement of titanium isotopic composition: Identification of correlated isotope anomalies in meteorites. J Anal At Spectrom. 2011;26:2197–2205. [Google Scholar]
- 25.Creech JB, Paul B. IsoSpike: Improved double‐spike inversion software. Geostand Geoanal Res. 2015;39:7–15. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.