Background
Medullary thyroid carcinoma (MTC) is one of the most challenging cancers. Epidemiological studies have shown that during the past 30 years neither a change in stage at diagnosis nor a significant improvement in survival has been achieved.1 Therefore, new diagnostic and therapeutic strategies are needed for early detection of metastases or disease recurrence and tumor growth control.
MTC is a neuroendocrine neoplasm deriving from thyroid parafollicular C cells. It accounts for nearly 5% to 10% of thyroid malignancies. The overall prognosis for MTC patients is relatively good. One-third of patients present with a locally invasive tumor or clinically apparent spread to the regional lymph nodes. Distant metastases are present in 13% of patients at initial diagnosis and portend a poor prognosis. Recurrent disease develops in approximately 50% of patients.2 Using a prior TNM classification system (7th edition), the 10-year survival rates for stages I, II, III, and IV are 100%, 93%, 71%, and 21%, respectively.
One-third of MTC cases result from a germline activating mutation in the proto-oncogene rearranged during transfection with a strong correlation between these mutations and their corresponding phenotypes.
Surgery is the only effective therapy in MTC. Undetectable basal serum calcitonin levels are a strong predictor of complete remission. When calcitonin levels are elevated, a careful evaluation and localization of postsurgical remnant tissue, early local recurrence, or metastases must be performed. Procalcitonin is an independent predictor of MTC progression. High procalcitonin-to-calcitonin ratios correlate with a high risk of progressive disease and shorter progression-free survival. Serum carcinoembryonic antigen is a useful biomarker of MTC, especially in poorly differentiated metastatic cases with lost ability to produce calcitonin. In some MTC patients with elevated postoperative calcitonin and short calcitonin doubling time, diagnostic and therapeutic options are limited due to unsuccessful disease localization with established imaging techniques, until the basal calcitonin level is at least 150 pg/ml.3–5 Therefore, a search for new targets and corresponding radionuclide imaging biomarkers is warranted.
There is still no efficient, universally recommended treatment regimen for advanced MTC. External beam therapy does not play a significant role. Conventional chemotherapy is of limited value. Newer agents such as irinotecan (a topoisomerase I inhibitor) and 17-AAG (a heat shock protein 90 inhibitor) are being evaluated in clinical trials.6
Tyrosine kinase inhibitors (TKIs) are currently being investigated (Supplementary material, Table S1). Vandetanib and cabozatinib have been approved for use in 2011 and 2012, respectively, by the Food and Drug Administration and European Medicines Agency. The low rate of partial responses to TKI therapy and absence of complete responses in any of the various trials of mono-therapy emphasize the need for new, more effective agents with acceptable toxicity.7
In clinical practice, evaluation of patients with MTC engages every available diagnostic option. Anatomic imaging (ultrasound, computed tomography [CT], or magnetic resonance imaging [MRI]) and numerous molecular imaging methods (positron emission tomography computed tomography and single-photon emission computed tomography–computed tomography [SPECT-CT]; Supplementary material, Table S2) have been used to localize recurrence or metastases of MTC. However, there are still many patients with negative imaging results.
The cholecystokinin 2 (CCK2) receptor is over-expressed in over 90% of MTC cases.8 Some of the CCK2 receptor–related peptides were tested in clinical pilot studies in humans. Receptor targeting was achieved to some extent in CCK2/gastrin receptor expressing tissues and, most importantly, in tumor tissue with a high tumor-to-background ratio. The CCK-2/gastrin receptors are also a promising target for peptide receptor radionuclide therapy (PRRT) as the peptide can be labeled with either a γ- or β-emitting radionuclide such as 90Y and 177Lu.
In an attempt to produce therapeutic options with CCK2/gastrin receptor–binding radiolabeled analogues, further research was initiated and coordinated within the European COST Action BM0607 “Targeted Radionuclide Therapy.”9,10 In these comparative studies, one derivative, namely DOTA-DGlu-DGlu-DGlu-DGlu-DGlu-DGlu-Ala-Tyr-Gly-Trp-Met-Asp-Phe-NH2 (CP04), showed the most promising characteristics: high metabolic stability and receptor affinity with a high and prolonged tumor uptake against low renal retention and was therefore selected for clinical evaluation.11 This derivative became a core substance for a new project.
The GRAN-T-MTC consortium operates within the ERA-NET on Translational Cancer Research (TRANSCAN) First Joint Transnational Call for Proposals 2011 (JTC 2011) on: “Validation of biomarkers for personalized cancer medicine.” It aims to develop a modern method of advanced MTC treatment, utilizing the phenomenon of overexpression of CCK2 receptor in this carcinoma. The project is a phase I study with the 111In-labelled gastrin analogue DOTA-(DGlu)6-Ala-Tyr-Gly-Trp-Met-Asp-Phe-NH2 (CP04). The positive results of the preclinical part of the project confirmed the possibility of administration of 111In-CP04 to humans.12,13 The trial is registered at www.clinicaltrials.gov (ID: NCT03246659) and has been granted European Union Drug Regulating Authorities Clinical Trials number: 2015-000 805-38. The project has been approved by the ethics committees in all centers participating in the clinical part of the project (Supplementary material, Table S3).
The primary aim of the study is to evaluate the safety of the intravenous administration of CP04, to assess the biodistribution and dosimetry of the CCK2/gastrin receptor ligand (111In-CP04) in cancerous and normal tissues of the human body, and to determine critical organs. The main secondary objectives are to evaluate the ability of CCK2/gastrin receptor ligand-scintigraphy to detect cancer lesions and to assess the ability of gelofusine co-injection.
The study consists of 2 phases: a preclinical part (Radiopharmaceutical development) and a clinical trial.
Radiopharmaceutical development (Work Package 1)
The first step was to establish a clinically useful formulation for the radiolabeled peptide CP04. CP04 was prepared in GMP quality, and tests regarding stability, radiolabeling, and toxicity were carried out. A ready-to-use 111In-CP04 kit formulation was prepared, and the Investigational Medicinal Product Dossier was submitted to the National Authorities of the collaborators.12,13
Clinical trial (Work Package 2)
Material and methods
A sample size of 20 to 25 recruited patients with progressive or metastatic MTC is considered appropriate for such a study, being a reasonable compromise to address the study’s purpose. The main inclusion criteria are: histologically documented MTC, presence of more than 1 distant or nodal metastases confirmed with either 18F-fluorodeoxyglucose positron emission tomography–computed tomography or contrast-enhanced CT or MRI, or calcitonin doubling time of less than 2 years if there is no anatomical evidence of disease, and the exclusion criteria: previous external beam radiation therapy within 2 years, pregnancy or breastfeeding, known hypersensitivity to gastrin analogues or gelofusine (Supplementary material, Table S4).
Two peptide amounts, both radiolabeled with 111In (200 +/− 10% MBq), were administered: a low amount (10 µg) was used as a safety step in the first applications of CP04. If its safety was assured, 50 µg (an amount also suitable for PRRT) was applied.
The clinical trial consisted of 2 phases. In phase 1A, the first 4 patients were administered with both 10 µg and 50 µg of 111In-CP04. In phase 1B, only the high peptide amount of 50 µg of 111In-CP04 was given to the next enrolled patient who was randomized to arm 1 or 2 without or with coadministration of gelofusine (a nephroprotective agent).
All baseline tumor-related sign and symptom assessments were performed before the start of the study. All eligible patients received a centrally assigned number. The tracer was injected within 14 days after the patient’s inclusion. According to the study protocol, subsequent tracer administrations were performed at intervals of at least 2 weeks, providing that no serious adverse events were observed.
Dosimetry was planned after each tracer injection including activity measurements in blood and urine, and a series of scintigraphy images: dynamic acquisitions of the abdominal region (0–30 minutes postinjection), planar whole-body acquisition (30–60 minutes 4, 24, and 48 hours postinjection), abdominal and neck or mediastinum SPECT-CT images (4–5 [optional] and 24 hours postinjection). Blood samples were collected for calcitonin and procalcitonin measurements.
Eight follow-up visits are planned up to 4 months after the tracer administration to assess the CP04 safety profile according to the Common Terminology Criteria for Adverse Events version 4.0.
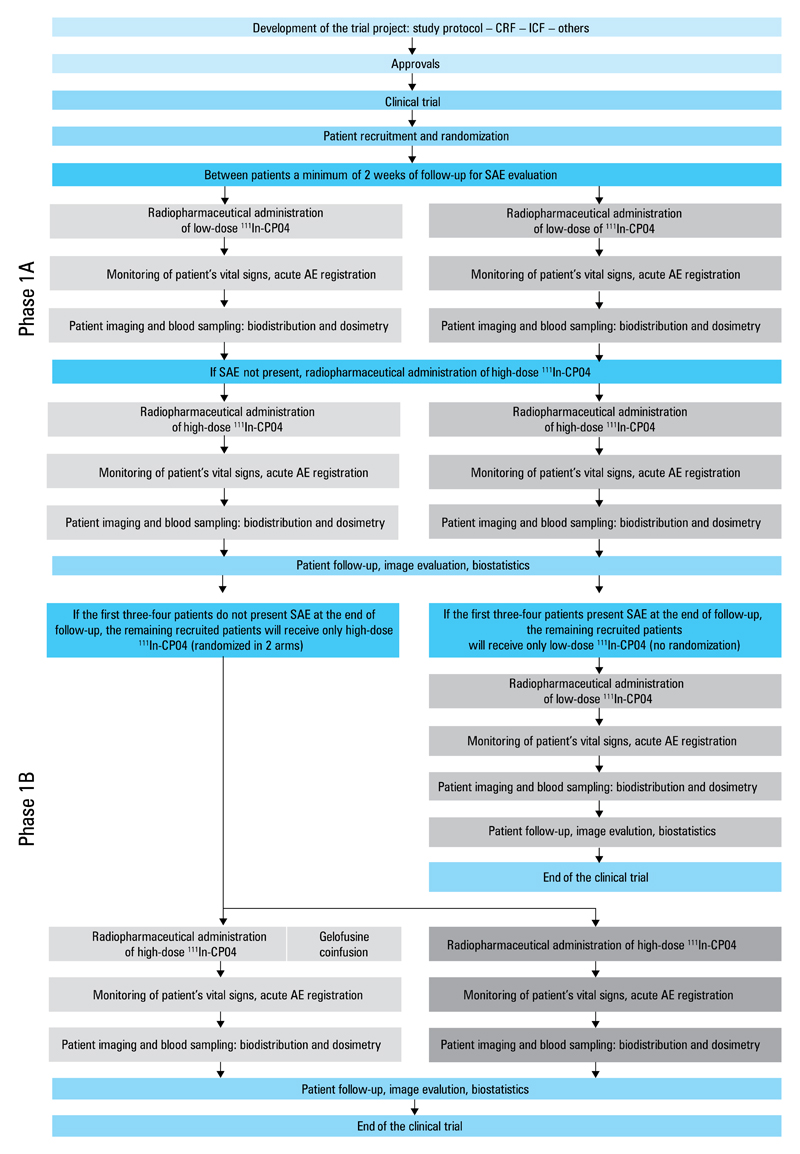
The study flowcharts are shown in Figure 1 and in Supplementary material (Table S3). Statistical analysis is done both on an intention-to-treat and per-protocol basis. The clinical trial was conducted according to international standards and the principles of the ICH Harmonized Tripartite Guidelines for Good Clinical Practice, with applicable local regulations and the Declaration of Helsinki.
Figure 1. Flowchart of the study protocol (phases 1A and 1B).
Abbreviations: AE, adverse event; CP04, DOTA-DGlu-DGlu-DGlu-DGlu-DGlu-DGlu-Ala-Tyr-Gly-Trp-Met-Asp-Phe-NH2; CRF, case report form; ICF, informed consent form; SAE, serious adverse event
Initial results
The completed preclinical part of the study showed that 111In CP04 can be prepared using a simple kit procedure, suitable for clinical use. According to the study protocol, each of the first 4 MTC patients enrolled in the study was injected with both peptide amounts (10 µg and 50 µg) of the gastrin analogue 111In-CP04. These initial examinations revealed that 111In-CP04 was safe after intravenous injection (no serious adverse events were reported) and that the MTC metastases could be detected with high sensitivity. Biodistribution and preliminary dosimetry data also showed 111In-CP04 as a promising radio-pharmaceutical for PRRT of advanced MTC cases.
Discussion
The main goal of the study is to translate the preclinical research results into the protocols confirming the usefulness of the peptide targeting CCK-2/gastrin receptors as a diagnostic and potentially also a therapeutic tool. Among the several new biomarkers investigated in recent years, the CP04 showed good stability and affinity to CCK-2/gastrin receptor in vitro, as well as favorable biodistribution and pharmacokinetic properties in vivo. For these reasons, it has been selected for the current study. CCK-2/gastrin receptors may become viable targets for radionuclide scintigraphy and PRRT, while nephrotoxicity and myelotoxicity will be minimized. In the last 2 decades, the somatostatin receptors were instrumental to implementing peptide receptor–mediated radionuclide diagnosis and therapy into clinical practice. It can be expected that CCK-2/gastrin receptor imaging will become also a valid diagnostic method for a specific and noninvasive staging and follow-up of patients with MTC.
In conclusion, the project represents the first step towards establishing a new, more efficient strategy for the diagnosis, early detection, and therapy of recurrent or metastatic MTC. CCK2/gastrin receptor may become a new target for radionuclide scintigraphy and PRRT (theranostic approach), with nephrotoxicity and myelotoxicity minimized by appropriate measures.
Supplementary Material
Supplementary material is available with the article at www.pamw.pl.
Acknowledgments
The project is funded by the European Commission under the Seventh Framework Programme (FP7) with the following national cofunding institutions: Ministry of Health (MoH), Italy; National Centre for Research and Development (NCBiR), Poland; Federal Ministry of Education and Research (BMBF), Germany; Austrian Science Fund (FWF), Austria; Ministry of Higher Education, Science and Technology (MHEST), Slovenia; and General Secretariat for Research and Technology, Ministry of Education, Life Long Learning and Religious Affairs (GSRT), Greece.
We would like to thank the ERA-NET on Translational Cancer Research and all national cofunding institutions for their support in the development of the project.
Footnotes
Conflict of interest: none declared.
References
- 1.Cupisti K, Wolf A, Raffel A, et al. Long-term clinical and biochemical follow-up in medullary thyroid carcinoma: a single institution’s experience over 20 years. Ann Surg. 2007;246:815–821. doi: 10.1097/SLA.0b013e31813e66b9. [DOI] [PubMed] [Google Scholar]
- 2.Miccoli P, Minuto MN, Ugolini C, et al. Clinically unpredictable prognostic factors in the outcome of medullary thyroid cancer. Endocr Relat Cancer. 2007;14:1099–1105. doi: 10.1677/ERC-07-0128. [DOI] [PubMed] [Google Scholar]
- 3.Machens A, Ukkat J, Hauptmann S, Dralle H. Abnormal carcinoembryonic antigen levels and medullary thyroid cancer progression: a multivariate analysis. Archives of Surgery. 2007;142:289–294. doi: 10.1001/archsurg.142.3.289. [DOI] [PubMed] [Google Scholar]
- 4.Wells SA, Jr, Asa SL, Dralle H, et al. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid. 2015;25:567–610. doi: 10.1089/thy.2014.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trimboli P, Seregni E, Treglia G, et al. Procalcitonin for detecting medullary thyroid carcinoma: a systematic review. Endocr Relat Cancer. 2015;22:R157–R164. doi: 10.1530/ERC-15-0156. [DOI] [PubMed] [Google Scholar]
- 6.Pinchot SN, Kunnimalaiyaan M, Sippel RS, Chen H. Medullary Thyroid Carcinoma: Targeted Therapies and Future Directions. J Oncol. 2009;2009 doi: 10.1155/2009/183031. 183031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grande E, Kreissl MC, Filetti S, et al. Vandetanib in advanced medullary thyroid cancer: review of adverse event management strategies. Adv Ther. 2013;30:945–966. doi: 10.1007/s12325-013-0069-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reubi JC, Waser B. Concomitant expression of several peptide receptors in neuroendocrine tumours: molecular basis for in vivo multireceptor tumour targeting. Eur J Nucl Med Mol Imaging. 2003;30:781–793. doi: 10.1007/s00259-003-1184-3. [DOI] [PubMed] [Google Scholar]
- 9.Von Guggenberg E, Rangger Ch, Sosabowski J, et al. Preclinical evaluation of radiolabelled DOTA-derivatized cyclic minigastrin analogs for targeting cholecystokinin receptor expressing malignancies. Mol Imaging Biol. 2012;14:366–375. doi: 10.1007/s11307-011-0506-2. [DOI] [PubMed] [Google Scholar]
- 10.Ocak M, Helbok A, Rangger Ch, et al. Comparison of biological stability and metabolism of CCK2 receptor targeting peptides, a collaborative project under COST BM0607. Eur J Nucl Med Mol Imaging. 2011;38:1426–1435. doi: 10.1007/s00259-011-1818-9. [DOI] [PubMed] [Google Scholar]
- 11.Peitl PK, Tamma ML, Kroselj M, et al. Stereochemistry of amino acid spacers determines the pharmacokinetics of 111In-DOTA-Minigastrin analogues for targeting the CCK2/gastrin receptor. Bioconjug Chem. 2015;26:1113–1119. doi: 10.1021/acs.bioconjchem.5b00187. [DOI] [PubMed] [Google Scholar]
- 12.Pawlak D, Rangger C, Kolenc-Peitl P, et al. From preclinical development to clinical application: Kit formulation for radiolabelling the minigastrin analogue CP04 with In-111 for a first-in-human clinical trial. Eur J Pharm Sci. 2016;85:1–9. doi: 10.1016/j.ejps.2016.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maina T, Konijnenberg MW, Kolenc-Peitl P, et al. Preclinical pharmacokinetics, biodistribution, radiation dosimetry and toxicity studies required for regulatory approval of a phase I clinical trial with (111)In-CP04 in medullary thyroid carcinoma patients. Eur J Pharm Sci. 2016;91:236–242. doi: 10.1016/j.ejps.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.