Abstract
Introduction
We conducted a double-blind randomised controlled trial in a remote-living Australian Aboriginal group at high risk for chronic disease to assess whether pharmacological treatment with angiotensin converting enzyme inhibitor (ACEi) could delay the onset of albuminuria, hypertension or diabetes in people currently free of those conditions.
Methods
Eligibility criteria in 2008 were age ≥18yr, blood pressure ≤140/90 mm/Hg, urinary albumin creatinine ratio (ACR) < 3.4 mg/mmol, normal levels of glycosylated haemoglobin, and, in females, infertility. A 2011 amendment allowed enrolment of fertile females using long-term contraception. “Treatment” was the ACEi perindopril arginine, or placebo, and participant events were ACR ≥3.4 mg/mmol and/or blood pressure >140/90 mm Hg and/or haemoglobin A1c >6.5%, and/or cardiovascular events. Results were analysed in 125 randomised participants who commenced treatment.
Results
Recruitment was low, especially of women, and dropout rates high: there were finally 60 and 65 people in the ACEi and placebo groups respectively. In females, there were no events among 10 in the ACEi group, versus 5 events among 17 in the placebo group, and longitudinal ACR, HbA1c and blood pressure levels supported probable benefit of ACEi. There was no benefit of ACEi in males, but a probable benefit on diabetes/hypertension events. With the genders combined, there was probable reduction of diabetes (zero vs 4 events, p = 0.068), and of diabetes or hypertension (zero vs 5 events, p = 0.037).
Discussion
In this high-risk population, ACEi probably delays development of albuminuria, diabetes and hypertension in females, and of non-ACR events overall. Repeat investigation with a larger sample size is warranted.
Keywords: Indigenous Australian, Albuminuria/hypertension, Diabetes, Randomised controlled trial, Angiotensin converting enzyme inhibitor (ACEi), Remote-living
Abbreviations: ACEi, angiotensin converting enzyme inhibitor; ACR, urinary albumin creatinine ratio; HbA1c, glycosylated haemoglobin A1c; RCT, randomised controlled trial
1. Introduction
In a high-risk remote-living Aboriginal group, we implemented a double-blind randomised controlled trial (RCT) to assess whether angiotensin converting enzyme inhibitor (ACEi) was effective in delaying the onset of albuminuria, hypertension or diabetes among adults “free” of these conditions.
Indigenous people in Australia have a high burden of chronic disease, which is most marked in those who live in the remote regions of the country [[1], [2], [3], [4]]. It is characterised by hypertension, kidney disease and type 2 diabetes which affect most of the population by middle age, with high rates of heart attacks, strokes, amputations, blindness, and kidney failure. Rates of hospitalizations for chronic diseases are very high, kidney failure has reached epidemic proportions, and governments are straining to meet the spiralling costs of dialysis treatment [5,6].
We conducted two screens, in 1992–1996 and 2004–2006, in the remote Tiwi, Aboriginal community, which both showed high rates albuminuria, hypertension and diabetes in adults and remarkable increases in rates with increasing age [7,8]. Among people who were free of the specific markers on the first screen, the estimated incidence between the screens of any or all of the endpoints was between 5.5 and 11 per 100 person years, with the most common marker being new onset albuminuria (unpublished data). We also showed, in a 3.5 year treatment program from late 1995 to November 1999, that treatment of subjects with albuminuria and hypertension with ACEi was associated with significant reductions in blood pressure (BP), and in rates of renal failure and all-cause natural deaths [9,10].
The high incidence of new-onset disease we observed, and the demonstrated benefit of treatment on existing disease, led us to question whether treatment with ACEi could prevent or delay subsequent disease onset in people who were, at a given point, disease free. We designed a randomised controlled trial to test this hypothesis.
2. Materials and methods
2.1. Recruitment
Volunteers from the remote community were potentially eligible for participation if they were ≥18 years of age, had no documented history of hypertension, diabetes or renal disease, and had BP ≤ 140/90 mm/Hg, urine albumin creatinine ratio (ACR) < 3.4 mg/mmol, and no diabetes on their most recent health check, and had no known contraindications to ACEi. A person was defined as diabetic if they had a medical history of diabetes, HbA1c >6.5% and/or they met the WHO criteria for diabetes on oral glucose challenge [11].
Reasons for exclusion included: a history of intolerance to ACEi; the presence of other diseases with potential to interfere with or contraindicate treatment; psychosis, or emotional, or intellectual issues that could invalidate informed consent or limit their ability to comply with protocol requirements; and, being a female of childbearing potential who was not using long-term contraception (sterilisation or a contraceptive implant).
Approval for the trial was obtained in 2006 from the community's Land Council and the Human Research Ethics Committees (HREC) of the Northern Territory Department of Health and the Menzies School of Health Research (06/34) and The University of Queensland (2006000671). However, the original approval ruled that potentially fertile women be excluded.
Beginning in June 2008, potentially eligible adult community members were identified from community health centre records and from results of the previous 2004–2006 community-wide chronic disease screening program [10].
Recruitment was delayed due to medicine supply problems, and enrolment, with informed consent, finally began in November 2008. Of 923 identified people contacted for an eligibility interview, 530 (57%) were deemed ineligible due to interim development of established disease or status as a potentially fertile woman. Of the remaining 393, 21 (2%) had died, 85 (9%) refused and 67 (7%) could not be contacted. Thus, only 220 (24%) appeared to be candidates for further eligibility testing. Of these, 160 candidates (33 females and 127 males) were found to be eligible and were subsequently enrolled.
Twenty-eight months into the trial, in February 2011, an ethics amendment was approved to allow enrolment of fertile women who were using long-term contraception. After a slow start, a further 9 women were enrolled between January and February 2012. A total enrolment of 169 adults, 127 (75%) men and 42 women (25%), was achieved.
2.2. Trial procedures
The intent was to randomise participants to the ACEi perindopril arginine or an inactive, visually identical placebo. Both were supplied by Servier. Participants were provided with 5 mg tablets (or placebo), and prescribed one tablet daily for the first month, then two tablets (10 mg) daily after one month and thereafter, where tolerated. If there was suspicion that the drug was not being tolerated, or if a serious adverse event occurred, treatment was immediately discontinued.
Administration of treatment, and collection of clinical measures and pathology specimens according to the trial's protocol, occurred from dedicated offices located near each of the Community's health centres.
The intended period of follow-up was 60 months with visits every 2 weeks for the first two months and monthly thereafter. Field staff managed the timing of visits with flexibility, accommodating unplanned events, as well as travel and weather disturbances. They also provided transport of participants to and from the field offices.
At each visit, participants returned their unused tablets, if there were any, at which time a count of tablets was recorded, returned tablets were disposed of and next month's allocation was issued. A reasonable degree of treatment adherence was considered to have occurred if participants took, on average, at least 50% of each treatment allocation.
Participants' data were monitored for departures from the normal range, side effects and adverse events as well as study outcomes. Day to day management was coordinated under the direction of the Chief Investigator. Oversight of the study was provided by an Advisory Committee and all adverse events were reportable to a Data Management Safety Committee. All data collection sheets were sent by airmail and fax to an independent data management unit, the Clinical Informatics and Data Management Unit, Department of Epidemiology and Preventive Medicine, Monash University.
A withdrawal was defined as an early exit that was not an outcome of interest. This occurred either when a participant was excluded by the Chief Investigator on grounds of medical safety or serious breach of trial protocol, or, when a participant withdrew their consent, becoming “lost to follow-up”.
2.3. Outcome event variables
The composite primary, clinical outcome was defined as hypertension (BP > 140/90 mm Hg) and/or albuminuria, ACR ≥3.4 mg/mmol, and/or HbA1c >6.5%, all these components were confirmed by one or two subsequent tests. Secondary outcomes were cardiovascular events, including myocardial infarction, cerebral or coronary ischaemic events and peripheral vascular events, including natural cardiovascular death.
The trial was powered to detect a 50% reduction in the number of primary outcome events with a treatment duration of up to 5 years, a 25% withdrawal rate and at least 114 people in each treatment arm. Calculations drew upon on the findings of the 2004–2006 health screen conducted in the same community in which 55% of adults, or about 600 people meeting the study eligibility criteria, had 5.5–11.2% annual incidence rates of the primary outcome (unpublished data). A minimum sample size requirement for the study was based upon the numbers of people having events in these earlier studies.
2.4. Statistical analysis
Analyses were conducted using Stata 14 software [StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP].
One hundred and twenty-five eligible participants who commenced treatment were included in analyses, utilising a modified intention-to-treat principle. Participants excluded from analyses (44/169) were those who did not commence treatment or those in whom ineligibility became apparent after randomisation.
Mean (SD), geometric mean (95%CI) or proportions were used to summarise variables. Group differences in continuous variables were tested with a t-test where variances were equal and distributions approximated normal, or a rank-sum test. A chi2 test was used for categorical variables. Skewed continuous variables were assessed using natural and log transformed versions.
Analyses were designed to assess the categorical combined outcome event. Survival time to event analyses were performed to estimate outcome incidence, and Kaplan–Meier survivor functions were tested for equality, using the log-rank test. Despite the intention to report Cox proportional hazard analyses, accounting for baseline age and or sex, it was not possible due to an insufficiency of outcome events. Analyses also examined the distributions of BP, ACR and HbA1c, as continuous variables.
The changes in systolic blood pressure (SBP), diastolic blood pressure (DBP), ACR and HbA1c were modelled with multilevel mixed-effects linear regression. Graphical representations of time-related trends in events were also inspected with locally weighted scatterplot smoothing (Lowess models) to assess the linearity of the relationships of outcome measures to time. All models were assessed with and without adjustment for combinations of the variables sex, baseline age and various degrees of treatment adherence. Those presented restrict follow-up time up to the first 36 months (including exits), so that group numbers were more robust.
3. Results
One hundred and sixty nine adults, 127 (75%) men and 42 women (25%), were randomised. Analyses were conducted on 125 participants (60 ACEi, 65 Placebo), after the exclusion of 44 who either took no treatment (n = 41), principally for reasons related to competitive football, drug and or alcohol use, mental health issues, voluntary or enforced absence from the community and lack of interest, or were falsely enrolled (n = 3), whose ineligibility became apparent after randomisation. The analysis group did not differ significantly by age, sex or randomisation to treatment arm, from those excluded (A 1). There was an apparent imbalance in females allocated to the treatment arms (ACEi/placebo), with 10/17 and 10/5 females in the analysis and non-analysis groups respectively (p = 0.065).
Baseline demographic, clinical and biochemical characteristics of the analysis group by treatment arm, are shown in Table 1. There were no significant differences.
Table 1.
Baseline characteristics, n-125 disease free adults, by treatment arm: Primary Prevention Trial, 2008–2013.
| Characteristic | ACEi N = 60 | Placebo N = 65 | P |
|---|---|---|---|
| Age, years; G mean (95% CI) | 31.7 (29.6–33.9) | 31.9 (29.7–34.1) | 0.909 |
| Sex; (M/F) | 50/10 | 48/17 | 0.198 |
| SBP, mm Hg (mean, SD) | 114.8 (11.0) | 113 (10.2) | 0.475 |
| DBP, mm Hg (median, IQR) | 73.0 (7.6) | 72.5 (9.1) | 0.705 |
| HbA1c, % (mean, SD) | 5.7 (0.2) n = 59 | 5.7 (0.2) n = 64 | 0.955 |
| ACR, mg/mmol; G mean (95% CI) | 0.61 (0.51–0.74) | 0.76 (0.63–0.92) | 0.116 |
| Weight, kg; G mean (95%CI) | 61.0 (57.8–64.3) n = 49 | 62.0 (59.0–65.10) n = 55 | 0.116 |
| Waist circumference, cms; median, IQR | 80.0 (74.0–91.0) n = 49 | 80.3 (74.3–90.0) n = 52 | 0.954 |
| Cholesterol, mmol/L; mean, SD | 5.1 (0.8) n = 60 | 4.9 (0.9) n = 63 | 0.422 |
| HDL, mmol/L; median, IQR | 1.2 (1.1–1.4) n = 60 | 1.2 (1.0–1.4) n = 63 | 0.326 |
| LDL, mmol/L; mean, SD | 3.1 (0.8) n = 59 | 3.1 (0.8) n = 63 | 0.788 |
| Triglycerides, mmol/g; G mean (95% CI) | 1.4 (1.2–1.7) n = 60 | 1.2 (1.1–1.4) n = 63 | 0.068 0.223 rsum |
Note: P = t-test, ranksum or chi2, SD = standard deviation, IQR = inter-quartile range, ACR = albumin creatinine ratio, SBP = systolic blood pressure, DBP = diastolic blood pressure, G mean = geometric mean.
The median length of an individual's participation was 2.5 years (0.08–4.25 years) in the ACEi arm and 3.0 years (0.08–4.27 years) in the placebo arm, they were not significantly different (p = 0.763). Sixty-five persons (52%) participated up to 3 years, 33 (26%) participated up to 1 year and 11 (8%) participated up to 3 months (not shown). Total person time on treatment was 138.7 person-years in the ACEi arm and 155.4 person-years in the placebo arm, an overall total of 294.1 years. Participation time did not differ by sex (p = 0.600), however, 7% (n = 9) of women were enrolled late in the trial. (Enrollment numbers at specified follow-up time points and a summary of follow-up time, by treatment arm and sex, are shown in A 2a and b). Adherence to the tablet regimen was variable, however; 73.3% (44/60) of participants in the ACEi arm took, on average, at least 50% of allocated treatment. There was no difference by sex; 70% of females (7/10) and 73% of males (44/60) had adherence of ≥50% (p = 0.548).
Fifty-nine participants (47%) withdrew from the trial prior to closeout for reasons other than a primary or secondary outcome of interest. Forty-seven participants (38%) were lost to follow-up, which commonly occurred when participants moved to another location, planned a pregnancy or played sport at an elite level. Three people (2.4%), all in the placebo arm, had a serious adverse event. None of these events was found to have a relationship to potential pharmacological toxicity, but they did result from study participation, and led to exclusion from the trial. Nine additional people (7%) were withdrawn due to the onset of medical conditions, all of which were clearly unrelated to study treatment. Six people had serious adverse events that did not necessitate withdrawal. There was no difference in the frequency of any of these categories by treatment arm. A 3a-b summarises the withdrawals by treatment arm and further describes the adverse events.
Sixteen primary or secondary outcomes of interest occurred among 15 participants (Table 2). Six people in the ACEi arm, all males, had 6 events (10% of participants), and the nine people in the placebo arm had 10 events (15.2% of participants). Specifically, 5 people in the ACEi arm developed elevated ACR and one developed myocardial infarction, while in the placebo arm, 4 developed diabetes, 1 hypertension, 3 elevated ACR, 1 myocardial infarction and 1 myocardial ischaemic event, with one of these people (a female) developing both diabetes and elevated ACR. All outcomes in the ACEi arm (all amongst males) were among people with adherence of at least 50% of allocated treatment.
Table 2.
Frequencies of primary and secondary outcome events, by category and treatment arm: Primary Prevention Trial, 2008–2013.
| Outcome | ACEi N = 60 |
Placebo N = 65 |
|---|---|---|
| Primary | ||
| Diabetes | 0 | 4 |
| Hypertension | 0 | 1 |
| Elevated ACR | 5 | 3 |
| Secondary | ||
| Cardiovascular event | 1 | 2 |
| Total | 6 (10%) | 10 (15.4%) |
Note: Elevated ACR = albumin creatinine ratio ≥3.4 mg/mmol; represents 16 events among 15 people, a combined elevated ACR and diabetes outcome occurred in one person in the placebo arm.
Notably, no females among 10 in the ACEi arm developed any outcomes (0%), while 5 among 17 in the placebo arm (29.4%) did.
There was a trend towards higher numbers of all other outcomes in the Placebo arm, with the exception of elevated ACR. We also examined the outcomes by treatment arm over time (provided in A 4); however, no persuasive pattern of difference emerged.
Incidence rates (unadjusted) for the outcome events, per 100 person-years, in females, males and females, and males together, are presented in Table 3A, Table 3B, Table 3CA–C. Notably, the absolute incidence rates in the placebo group, and the 2-fold differences between males and females, are entirely compatible with the (unpublished) data collected in the natural history phase on which the sample size for this trial was originally estimated.
Table 3A.
Incidence rates of outcomes per event by treatment arm: Primary Prevention Trial females, 2008–2013.
| Outcome | ACEi N = 10 FU: 17.3 py |
Placebo N = 18 FU: 45 py |
P rates ACEi vs placebo |
|---|---|---|---|
| # events; rate (CI) per 100 py | # events; rate (CI) per 100 py | ||
| Diabetes | 0; 0 | 3; 6.7 (2.2–20.7) | 0.382 |
| Hypertension | 0; 0 | 0; 0 | NA |
| Elevated ACR | 0; 0 | 1; 2.2 (0.3–15.8) | 0.547 |
| Cardiovascular | 0; 0 | 1; 2.2 (0.3–15.8) | 0.706 |
| Non-ACR | 0; 0 | 4; 8.9 (3.3–23.7) | 0.342 |
| Any/all | 0; 0 | 5; 11.1 (4.6 – 26.7) | 0.251 |
Table 3B.
Incidence rates of outcomes per event by treatment arm: Primary Prevention Trial males, 2008–2013.
| Outcome | ACEi N = 50 FU: 121.4 py |
Placebo N = 48 FU: 112.7 py |
P rates ACEi vs placebo |
|---|---|---|---|
| # events; rate (CI) per 100 py | # events; rates (CI) per 100 py | ||
| Diabetes | 0; 0 | 1; 0.9 (0.1–6.3) | 0.289 |
| Hypertension | 0; 0 | 1; 0.9 (0.1–6.3) | 0.239 |
| Elevated ACR | 5; 4.1 (1.7–9.9) | 2; 1.8 (0.5–7.1) | 0.283 |
| Cardiovascular | 1; 0.8 (0.1–5.9) | 1; 0.9 (0.1–6.3) |
0.950 |
| Non-ACR | 1; 0.8 (0.1–5.9) | 3; 2.7 (0.9–8.3) | 0.246 |
| Any/all | 6; 4.9 (2.2 – 11.0) | 5; 4.4 (1.9– 10.7) | 0.873 |
Table 3C.
Incidence rates of outcomes per event by treatment arm: Primary Prevention Trial females and males, 2008–2013.
| Outcome | ACEi N = 60 FU: 138.7 py |
Placebo N = 66 FU: 157.7 py |
P rates ACEi vs placebo |
|---|---|---|---|
| # events; rate (CI) per 100 py | # events; rate (CI) per 100 py | ||
| Diabetes | 0; 0 | 4; 2.5 (1.0–6.8) | 0.072 |
| Hypertension | 0; 0 | 1; 0.6 (0.09–4.5) | 0.330 |
| Elevated ACR | 5; 3.6 (1.5–8.7) | 3; 1.9 (0.6–5.9) | 0.357 |
| Cardiovascular | 1; 0.7 (0.1–5.1) | 2; 1.3 (0.3–5.1) | 0.670 |
| Non-ACR | 1; 0.7 (0.1–5.1) | 7; 4.4 (2.1–9.3) | 0.060 |
| Any/all | 6; 4.3 (1.9 – 9.6) | 10; 6.3 (3.4 -11.8) | 0.500 |
Note: ACEi, angiotensin converting enzyme inhibitor; P, log-rank tests of equality calculated from Kaplan-Meier survival functions (unadjusted); FU, follow-up period; py, person-years; CI, 95% confidence interval; #, number; ACR, albumin creatinine ratio mg/mmol.
The tables show that diabetes, hypertension and secondary outcome events were more common in the placebo arm in both females and males (3A,B). In combination, these “non-ACR” events occurred at a rate of 0.7 (0.1–5.1) per 100 person-years in the ACEi arm, and at a rate of 4.4 (2.1–9.3) per 100 person-years in the placebo arm (3C). The difference in these rates approached significance (P = 0.060). There was also a higher, although non-significant (p = 0.500), rate of a combined event of all primary and secondary outcomes in the placebo arm among all participants (3C). None of the ten females in the ACEi arm experienced any event (0%), in contrast to 5 out of 17 females (28%) in the placebo arm, at a rate of 11.1 (4.6–26.7) per 100 person-years: however, the difference was not significant (P = 0.251) (3A).
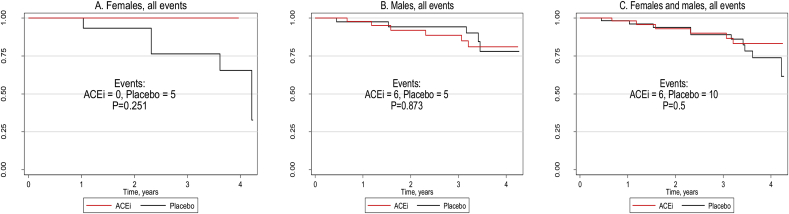
Kaplan-Meier survival curves of the outcome events combined, by treatment arm, in females, males, and females and males together (Fig. 1A–C), highlight the wider discrepancy in the female rather than the male rates.
Fig. 1.
A–C. Kaplan-Meier survival curves of all events by treatment arm and sex: Primary Prevention Trial, 2008-2013
Note: ACEi, angiotensin converting enzyme inhibitor; P, log-rank tests of equality calculated from Kaplan-Meier survival functions (unadjusted).
Cox proportional hazards models for a combined outcome event in all participants suggested a lower rate of events in the ACEi arm, although non-significant (not shown). It was not possible to derive estimates for females separately as there were no events among females in the ACEi arm.
Changes over time (up to 36 months of follow-up) in the outcome measures of HbA1c, ACR, SBP and DBP, without additional adjustment or exclusion of outlying values, are summarised in A 5. In females there was no significant difference between the ACEi and placebo arms for any of these outcomes, however, they all, particularly ACR, had a non-significant tendency to be at a lower level in the ACEi arm.
Males in the treatment arm showed no benefit for HbA1c, and a non-significant trend towards benefit in SBP.
Adjustment for age at randomisation did not alter any findings.
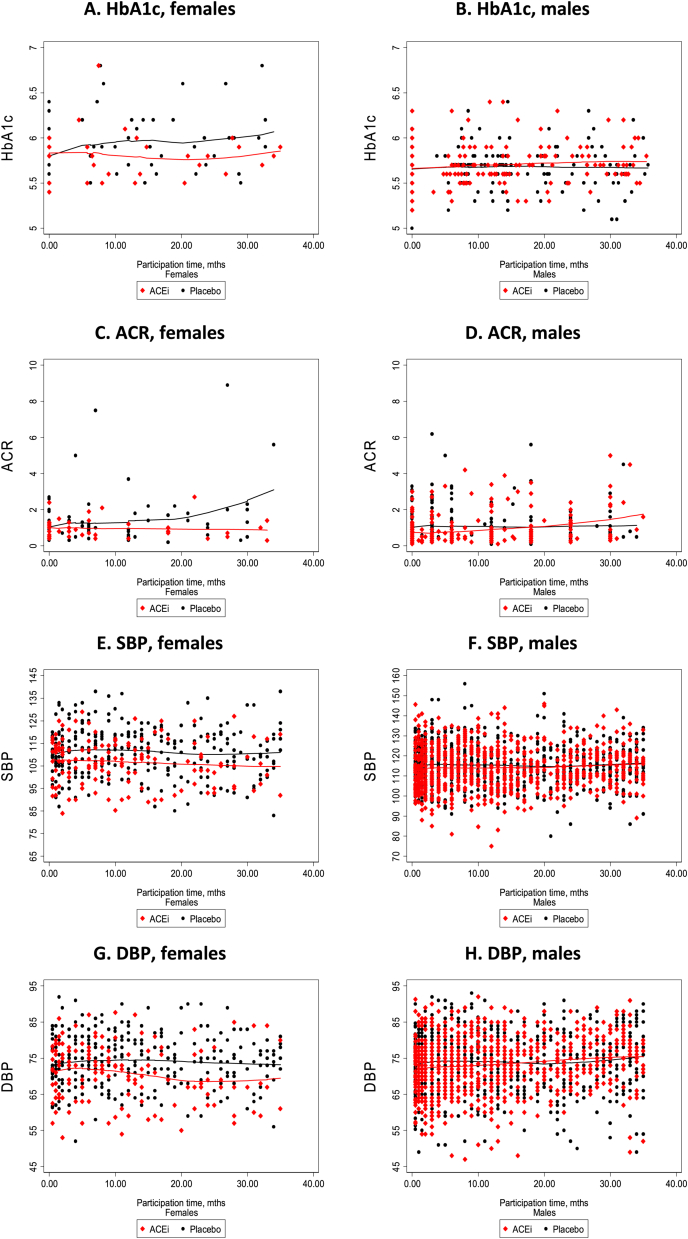
Lowess modelling, without adjustment (see Fig. 2A–H), lends support to these findings and indicates that the relationships may not be linear.
Fig. 2.
A–H., HbA1c, ACR, SBP and DBP levels over time, by sex, and treatment group: Primary Prevention Trial, 2008-2013
Note: Graphs show locally weighted scatterplot smoothing of unadjusted estimates for the period of up to the 36 month of follow-up; HbA1c, glycosylated haemoglobin A 1c; ACR, albumin creatinine ratio; SBP, systolic blood pressure (mm/Hg); DBP, diastolic blood pressure (mm/Hg).
4. Discussion
This was the first randomised controlled trial of pharmaceutical prevention of albuminuria, hypertension or diabetes in the setting of a high-risk remote-living Australian Aboriginal group. It was conducted in a very challenging environment with multiple logistic hurdles.
Delayed ethics approval for commencement of the trial led to a prolonged recruitment period in which the pool of potentially eligible participants was reduced by new incidence of interim disease, reflecting the high rates of disease experienced by the community. Additionally, numbers of enrolled females were lower than anticipated, due to delay of approval for the enrolment of fertile women who were using long-term contraception, until the final two years of the trial. In remote settings Aboriginal women, compared to men, have higher levels of elevated ACR and higher incidence of new onset ACR up through early adult life [12] and females also have significantly higher levels of end stage kidney disease leading to RRT [13,14]. Exclusion of potentially fertile women at the outset of the trial (and the inference that they were not capable of managing their own fertility) created a negativity towards the trial in both sexes, and contributed to the high withdrawal rate of 47%, which was higher than the estimate of 25% used in power calculations. The recruitment goal of ≥114 people in each arm was not achieved. Based upon the minimum requirement, and among those analysed, there was a 14% deficit in male and a 76% deficit in female enrolment.
Analyses were stymied by their lack of power, related to the small unbalanced numbers of participants and outcomes, as well as to recruitment biases and considerable periods of poor quality adherence data. Field Staff reported instances of periodic medicine supply failure due to weather events and tablet production problems, potential participant awareness of their randomisation due to cosmetic differences in tablets, and questionable accuracy of returned tablet count registrations.
Elevated ACR was the most frequent outcome, but it was not significantly more frequent in the placebo than the treatment arm. A possible treatment benefit in terms of a combined diabetes, hypertension outcome, and, arguably, on cardiovascular events, however, does lend support to our hypothesis, as do the observed tendencies for lower SBP and HbA1c in the ACEi arm during follow-up. In 2001 the HOPE study reported that new onset of diabetes, using elevated HbA1c as the diagnostic marker, was reduced in individuals taking the ACEi, Ramipril, compared to those on Placebo [15].
5. Conclusion
We present a catalogue of events describing an RCT of ACEi in a remote Aboriginal Australian community. There was sub-optimal recruitment, a gender imbalance, a small sample size, and subsequent difficulty in interpreting the data. Nonetheless, there was a suggestion of benefit of ACEi in females for all endpoints, and a trend towards benefit on non-ACR endpoints when both sexes were examined together. In view of the huge burden of chronic disease in these communities, consideration should be given to repeating some aspects of such a trial under better conditions.
Trial registration
Australian New Zealand Clinical Trials Registration, Number 12608000371392.
Ethics
Ethical approval for the study was granted by the human research ethics committees of the Northern Territory Department of Health and Menzies School of Health Research, The University of Queensland, and the Tiwi Land Council.
Funding
This work has been supported by the Australian Government National Health and Medical Research Council (NHMRC) Australia Research Fellowship to Hoy, 2008–2012 (grant number 511081); a grant from the Colonial Foundation of Australia, 2008–2011; and the NHMRC Centre of Research Excellence in Chronic Kidney Disease in Australia, 2014- current (grant number 1079502). These funding sources had no involvement in the conduct of the research and/or the preparation of this article.
Acknowledgements
We thank the participants and the Tiwi Land Council for their participation. Servier supplied the active agent, perindopril arginine and placebo. Members of the Study's Advisory Committee were, Prof Stephen Colagiuri, Prof David Harris, Prof Carmel Hawley, Dr Rosemary Lee, Assoc Prof Tim Matthew, Mrs Noelene Swanson, and Assoc Prof Mark Thomas; and Members of the Data Safety and Monitoring Committee were E/Prof Lawrie Beilin (Chair), Dr Nick Andrianopoulos, Mr Bernard Tipiloura, and Prof Andrew Tonkin. We thank the Menzies School of Health Research based Study Staff: Gai Alcock, Carl Bourke, Jennifer Godfrey, David Guy, Marietta Guy, Carl Heaslop, Alayne Montz; Cecily Nixon; Natasha Pilakui, Ambrose Portaminni, Suresh Sharma, and Barry Ullungura; and the Menzies School of Health Research for accommodating and supporting the Study Team.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.conctc.2019.100323.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Australian Institute of Health and Welfare 2014 Australia’s health . AIHW; Canberra: 2014. Australia's Health Series No. 14. Cat. No. AUS 178. [Google Scholar]
- 2.Australian Bureau of Statistics . AIHW; Canberra: 2014. Australian Aboriginal and Torres Strait Islander Health Survey: Biomedical Results. 2012–13. 4727.0.55.003. [Google Scholar]
- 3.Hoy W.E., Kondalsamy Chennakesavan S., McDonald S.P., Cass A., Singh G.R., Bertram J.F., Hughson M.D. Chronic kidney disease in Aboriginal Australians. In: El Nahas M., editor. Kidney Disease in Ethnic Minorities and the Developing World. Taylor & Francis; New York, U.S: 2005. pp. 305–333. [Google Scholar]
- 4.Hoy W.E., Mott S.A., McDonald S. An expanded nationwide view of chronic kidney disease in Aboriginal Australians. Nephrology (Carlton) 2016;21(11):916–922. doi: 10.1111/nep.12798. PMID:27075933. Erratum, Jan 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoy W.E., Kondalsamy-Chennakesavan S., Wang Z., Briganti E., Shaw J., Polkinghorne K., Chadban S., The AusDiab Study Group Quantifying the excess risk for proteinuria, hypertension and diabetes in Australian Aborigines: comparison of profiles in three remote communities in the Northern Territory with those in the AusDiab study. Aust N Z J Public Health. 2007 Apr;31(2):177–183. doi: 10.1111/j.1753-6405.2007.00038.x. [DOI] [PubMed] [Google Scholar]
- 6.ANZDATA Registry . Chapter 12: End Stage Kidney Disease Among Indigenous Peoples of Australia and New Zealand. Australia and New Zealand Dialysis and Transplant Registry, Adelaide, Australia. 2017. 2016. http://www.anzdata.org.au 39th Report. Available at: [Google Scholar]
- 7.Wang Z., Hoy W.E. Albuminuria and risk of developing diabetes in Aboriginal Australians. Int. J. Epidemiol. 2006;35(5):1331–1335. doi: 10.1093/ije/dyl115. [DOI] [PubMed] [Google Scholar]
- 8.Wang Z., Hoy W.E. The predictive value of albuminuria for renal and nonrenal deaths over 14 years follow-up in a remote aboriginal community. Clin. Kidney J. 2012;0:1–7. doi: 10.1093/ckj/sfs125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoy W.E., Baker P., Kelly A., Wang Z. Reducing premature death and renal failure in Australian Aborigines: results of a community-based treatment program. Med. J. Aust. 2000;172:473–478. [PubMed] [Google Scholar]
- 10.Hoy W.E., Wang Z., Kelly A., Baker P.R.A. Sustained reduction in renal failure and cardiovascular deaths from a systematic treatment program in an Australian Aboriginal community. Kidney Int. 2003;63(Suppl 83):S66–S73. doi: 10.1046/j.1523-1755.63.s83.14.x. [DOI] [PubMed] [Google Scholar]
- 11.American Diabetes Association Standards of care for diabetes-2009. Diabetes Care. 2009;32(suppl 1):513–561. [Google Scholar]
- 12.Hoy W.E., Kincaid-Smith P., Hughson M.D., Fogo A., Sinniah R., Dowling J., Samuel T., Mott S.A., Douglas-Denton R., Bertram J.F. Invited review for world kidney forum, CKD in aboriginal Australians. Am. J. Kidney Dis. 2010;56(5):983–993. doi: 10.1053/j.ajkd.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 13.Hoy W.E., Mott S.A., McDonald S. An expanded nationwide view of chronic kidney disease in Aboriginal Australians. Nephrology (Carlton) 2016;21(11):916–922. doi: 10.1111/nep.12798. PMID:27075933. Errata, Jan 2017 & Jan 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoy W.E., Normal R.J., Hayhurst B.G., Pugsley D.J. A heath profile of adults in a Northern Territory Aboriginal community: with an emphasis on preventable morbidities. Aust. N Z J Public Health. 1997;21:121–126. doi: 10.1111/j.1467-842x.1997.tb01670.x. [DOI] [PubMed] [Google Scholar]
- 15.Gerstein H.C. Reduction of cardiovascular events and microvascular complications in diabetes with ACE inhibitor treatment: HOPE and MICRO-HOPE. Diabetes Metab Res Rev. 2002;18:S82–S85. doi: 10.1002/dmrr.285. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.