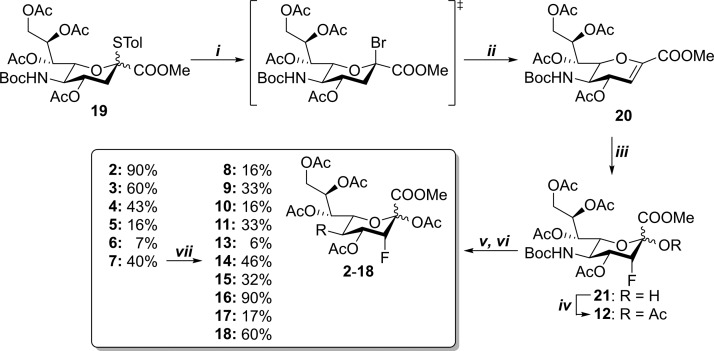
Scheme 1. Synthesis of C-5-Modified ST Inhibitors.
(i) Br2, DCM, r.t., 2.5 h; (ii) TEA, DCM, r.t., 16 h, 78% (over two steps); (iii) Selectfluor, 1:3 H2O/DMF, 60 °C, 3 h, 72% (based on recovery); (iv) Ac2O, py, r.t., 48 h, 95%; (v) TFA, DCM, H2O, r.t., 2 h; (vi) activated acyl substituents (A), TEA, DCM, r.t.; 2: Side product in a reaction of 12 → 11 90%; 3: A = chloroacetyl chloride, 16 h, 60%; 4: A = acetoxyacetyl chloride, 16 h, 43%; 5: A = azidoacetic acid N-hydroxysuccinimide (NHS) ester, 23 h, 16%; 6: A = 4-pentynoic acid NHS ester, 16 h, 7%; 7: A = N-propargyloxycarbonyl-succinimide, 15 h, 40%; 8: A = allyl chloroformate, 21.5 h, 16%; 9: A = methyl chloroformate, 16 h, 33%; 10: A = ethyl chloroformate, 16 h, 16%; 11: A = isobutyl chloroformate, 16 h, 33%; 13: A = benzyl chloroformate, 21.5 h, 6%; 15: A = n-butyl chloroformate, 16 h, 32%; 16: A = 2-methoxymethyl chloroformate, 16 h, 90%; 17: A = 2,2,2-trichloroethoxycarbonyl chloroformate, 16 h, 17%; 18: A = 2-fluoroethyl chloroformate, 16 h, 60%; and (vii) 14: benzyl azide, TBTA, CuI, Cu, DMF, H2O, tBuOH, r.t., 16 h, 46%.